217247
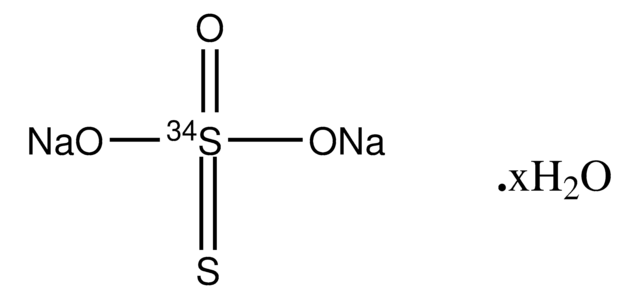
Sodium thiosulfate pentahydrate
ACS reagent, ≥99.5%
About This Item
crystals
pellets
Produtos recomendados
grau
ACS reagent
Nível de qualidade
Agency
suitable for EPA 1621
suitable for ISO 25101
suitable for SM 2310
suitable for SM 2320
suitable for SM 4500 - NH3
Ensaio
≥99.5%
Formulário
crystalline powder
crystals
pellets
Impurezas
≤0.002% N compounds
≤0.005% insolubles
pH
6.0-8.4 (25 °C, 5%)
solubilidade
water: soluble(lit.)
traços de ânion
S2-: passes test
SO42- and SO32-: ≤0.1%
traços de cátion
N: ≤0.002%
cadeia de caracteres SMILES
O.O.O.O.O.[Na+].[Na+].[O-]S([O-])(=O)=S
InChI
1S/2Na.H2O3S2.5H2O/c;;1-5(2,3)4;;;;;/h;;(H2,1,2,3,4);5*1H2/q2*+1;;;;;;/p-2
chave InChI
PODWXQQNRWNDGD-UHFFFAOYSA-L
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Descrição geral
Aplicação
- Thioester derivatives via one-pot two-step reactions with organic halides and aryl anhydrides.
- Sulfur nanoparticles using F. benghalensis leaf extract which acts as a reducing and capping agent.
- Unsymmetrical heteroaryl thioethers via multicomponent reaction with heteroaryl chlorides and alcohols.
It can also be used in the fabrication of microencapsulated phase change materials for thermal energy storage applications.
Características e benefícios
Código de classe de armazenamento
13 - Non Combustible Solids
Classe de risco de água (WGK)
WGK 1
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica