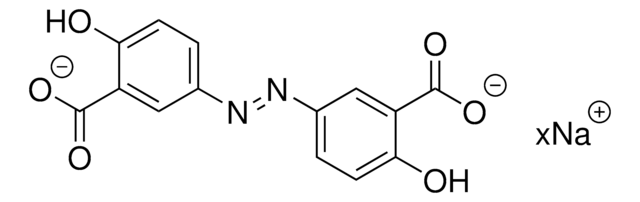
O0146010
Olsalazine sodium for performance test
European Pharmacopoeia (EP) Reference Standard
Sinônimo(s):
4,4′-Dihydroxyazobenzene-3,3′-dicarboxylic acid disodium salt, 5,5′-Azobis(salicylic acid, sodium salt), Mordant Yellow 5
About This Item
Produtos recomendados
grau
pharmaceutical primary standard
família API
olsalazine
fabricante/nome comercial
EDQM
aplicação(ões)
pharmaceutical (small molecule)
formato
neat
temperatura de armazenamento
2-8°C
InChI
1S/C14H10N2O6.2Na/c17-11-3-1-7(5-9(11)13(19)20)15-16-8-2-4-12(18)10(6-8)14(21)22;;/h1-6,15,17H,(H,19,20)(H,21,22);;/q;2*+1/p-2/b16-8-;;
chave InChI
QQWFSVYVHLECFP-XBPUGJBTSA-L
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Aplicação
Embalagem
Outras notas
produto relacionado
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Certificados de análise (COA)
Lamentamos, não temos COA para este produto disponíveis online no momento.
Se precisar de ajuda, entre em contato Atendimento ao cliente
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica