80757
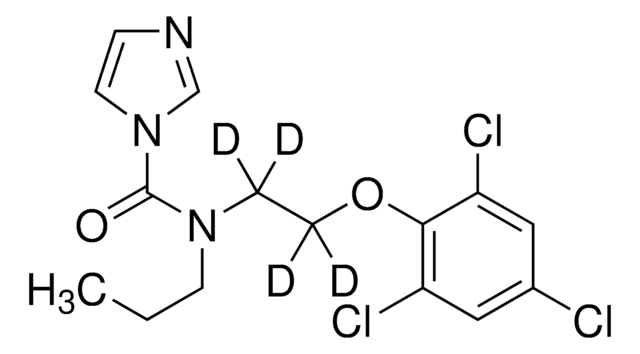
Propamocarb-(propyl-d7)
PESTANAL®, analytical standard
Sinônimo(s):
Propamocarb-d7
Faça loginpara ver os preços organizacionais e de contrato
About This Item
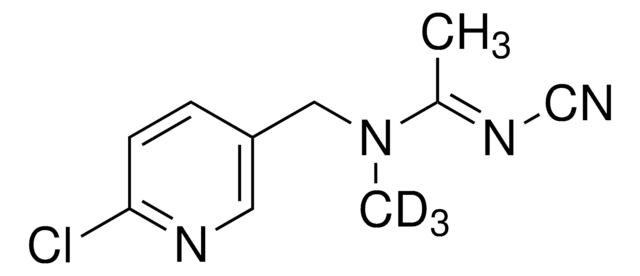
Fórmula empírica (Notação de Hill):
C9D7H13N2O2
Peso molecular:
195.31
Número MDL:
Código UNSPSC:
41116107
ID de substância PubChem:
NACRES:
NA.24
Produtos recomendados
grau
analytical standard
Nível de qualidade
linha de produto
PESTANAL®
Ensaio
≥98.0% (GC)
prazo de validade
limited shelf life, expiry date on the label
aplicação(ões)
agriculture
Formato
neat
temperatura de armazenamento
2-8°C
cadeia de caracteres SMILES
CN(C)CCCNC(OC([2H])([2H])C([2H])([2H])C([2H])([2H])[2H])=O
InChI
1S/C9H20N2O2/c1-4-8-13-9(12)10-6-5-7-11(2)3/h4-8H2,1-3H3,(H,10,12)/i1D3,4D2,8D2
chave InChI
WZZLDXDUQPOXNW-GZAMCDGTSA-N
Descrição geral
Propamocarb-(propyl-d7) is an isotope-labeled analog of systemic fungicide propamocarb, wherein propyl protons are substituted by deuterium.
Aplicação
Propamocarb-(propyl-d7) may be used as a deuterated internal standard to quantify the pesticide analytes in foods of plant origin by two-dimensional liquid chromatography-tandem mass spectrometry (LC-MS/MS).
Isotope-labeled compounds are increasingly used in isotope dilution mass spectrometry (IDMS) for the quantitative analysis of pesticides. The major advantage of using this technique is that the isotope-labeled compounds have nearly the same physical properties as their non-labeled counterpart analogs, and thus show identical behavior during the workup and sample preparation process. This helps in overcoming the problems of matrix effects generally encountered in the usual LC-MS/GC-MS analysis potentially resulting in biased results. By spiking the sample before workup with its isotope labeled analog, the loss of analyte that leads to matrix effects can be determined and compensated.
Isotope-labeled compounds are increasingly used in isotope dilution mass spectrometry (IDMS) for the quantitative analysis of pesticides. The major advantage of using this technique is that the isotope-labeled compounds have nearly the same physical properties as their non-labeled counterpart analogs, and thus show identical behavior during the workup and sample preparation process. This helps in overcoming the problems of matrix effects generally encountered in the usual LC-MS/GC-MS analysis potentially resulting in biased results. By spiking the sample before workup with its isotope labeled analog, the loss of analyte that leads to matrix effects can be determined and compensated.
Informações legais
PESTANAL is a registered trademark of Merck KGaA, Darmstadt, Germany
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Oral
Código de classe de armazenamento
10 - Combustible liquids
Classe de risco de água (WGK)
WGK 2
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Simultaneous determination of pesticides, mycotoxins, tropane alkaloids, growth regulators, and pyrrolizidine alkaloids in oats and whole wheat grains after online clean-up via two-dimensional liquid chromatography tandem mass spectrometry
Urban M, et al.
Journal of environmental science and health. Part. B, Pesticides, food contaminants, and agricultural wastes, 54(2), 98-111 (2019)
Quick method for the analysis of numerous highly polar pesticides in foods of plant origin via LC-MS/MS involving simultaneous extraction with methanol (QuPPe-method)
Anastassiades M, et al.
EU Reference Laboratie for Residue of Pesticides, 9, 1-60 (2015)
Simultaneous determination of pesticides, mycotoxins, and metabolites as well as other contaminants in cereals by LC-LC-MS/MS
Kresse M, et al.
Journal of Chromatography. B, Biomedical Applications, 1117(2), 86-102 (2019)
Quick method for the analysis of numerous highly polar pesticides in foods of plant origin via LC-MS/MS involving simultaneous extraction with methanol (QuPPe-method)
Anastassiades M, et al.
Chemosphere, 1117(2), 86-102 (2019)
Rosalía López-Ruiz et al.
Chemosphere, 226, 36-46 (2019-03-27)
In this study, fenamidone, propamocarb and their transformation products were monitored in cherry tomato, cucumber, and courgette samples. A mixture of both compounds, which have different physico-chemical characteristics, are commercially available (Consento®). For analysis, ultra high-performance liquid chromatography coupled to
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica