About This Item
Fórmula linear:
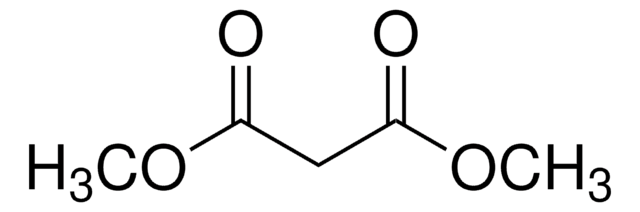
CH2(COOCH3)2
Número CAS:
Peso molecular:
132.11
Beilstein:
774261
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
densidade de vapor
>1 (vs air)
Nível de qualidade
grau
purum
Ensaio
≥96.0% (GC)
índice de refração
n20/D 1.413 (lit.)
n20/D 1.413
p.e.
180-181 °C (lit.)
pf
−62 °C (lit.)
densidade
1.156 g/mL at 25 °C (lit.)
grupo funcional
ester
cadeia de caracteres SMILES
COC(=O)CC(=O)OC
InChI
1S/C5H8O4/c1-8-4(6)3-5(7)9-2/h3H2,1-2H3
chave InChI
BEPAFCGSDWSTEL-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2
Código de classe de armazenamento
10 - Combustible liquids
Classe de risco de água (WGK)
WGK 1
Ponto de fulgor (°F)
185.0 °F - closed cup
Ponto de fulgor (°C)
85 °C - closed cup
Equipamento de proteção individual
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
P Gimsing et al.
American journal of hematology, 49(2), 121-130 (1995-06-01)
The cobalamin metabolism in chronic myelogenous leukemia (CML) was evaluated in 18 newly diagnosed and untreated patients by formiminoglutamic acid (FiGlu) and methyl malonic acid excretion (MMA) tests. A deoxyuridine (dU) suppression test of bone marrow cells was compared in
Jeroen S Dickschat et al.
Chembiochem : a European journal of chemical biology, 5(6), 778-787 (2004-06-03)
The volatiles emitted from cell cultures of myxobacterium Myxococcus xanthus were collected by use of a closed-loop stripping apparatus (CLSA) and analyzed by GC-MS. Two new natural products, (S)-9-methyldecan-3-ol ((S)-1) and 9-methyldecan-3-one (2), were identified and synthesized, together with other
Violeta Iosub et al.
The Journal of organic chemistry, 75(5), 1612-1619 (2010-02-06)
A convenient and versatile enantioselective synthesis of biologically important alpha-quaternary amino acid derivatives was based on the sequential double alkylation or arylation of dimethyl malonate, followed by desymmetrization with porcine liver esterase (PLE) and Curtius rearrangement. The PLE-mediated hydrolysis of
R Padmakumar et al.
Analytical biochemistry, 214(1), 318-320 (1993-10-01)
A rapid and high-yield synthesis of methylmalonyl coenzyme A is reported. The two-step procedure involves preparation of the thiophenyl ester of methylmalonic acid using dicyclohexylcarbodiimide as a condensing agent, followed by transesterification with coenzyme A, to yield methylmalonyl coenzyme A
H Dongmei et al.
Preparative biochemistry & biotechnology, 30(3), 231-240 (2000-08-05)
5-Methoxytryptamine and L-tryptophan methyl ester were acylated with malonic acid, dimethyl malonate, or succinic anhydride to produce the corresponding N,N'-dicarbonyltryptamine derivatives. The analgesic activity was evaluated by the tail flick test. All of the compounds exhibited desirable analgesic potency. This
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica