424633
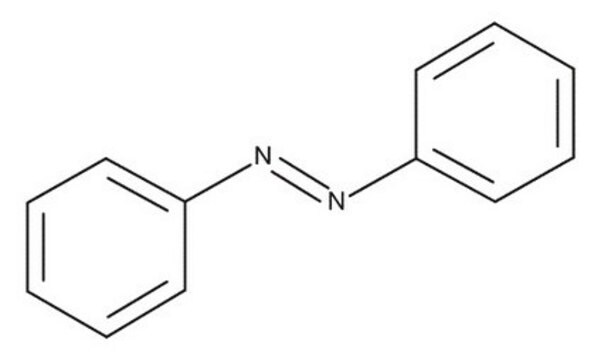
Azobenzene
98%
Sinônimo(s):
1,2-Diphenyldiazene; Diphenyldiazene
About This Item
Produtos recomendados
pressão de vapor
1 mmHg ( 104 °C)
Nível de qualidade
Ensaio
98%
forma
powder or crystals
temperatura de autoignição
890 °F
pb
293 °C (lit.)
pf
65-68 °C (lit.)
densidade
1.09 g/mL at 25 °C (lit.)
aplicação(ões)
diagnostic assay manufacturing
hematology
histology
temperatura de armazenamento
room temp
cadeia de caracteres SMILES
c1ccc(cc1)\N=N\c2ccccc2
InChI
1S/C12H10N2/c1-3-7-11(8-4-1)13-14-12-9-5-2-6-10-12/h1-10H/b14-13+
chave InChI
DMLAVOWQYNRWNQ-BUHFOSPRSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Descrição geral
Aplicação
As human tissue is translucent to red and near-infrared light but opaque to blue and UV light, Azobenzene is important in medicine and photopharmacology for applications that involve shifting the absorptions of both trans and cis isomers of azobenzene to longer wavelengths.
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 1B - Muta. 2 - STOT RE 2
Código de classe de armazenamento
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
212.0 °F - closed cup
Ponto de fulgor (°C)
100.0 °C - closed cup
Equipamento de proteção individual
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica