31550
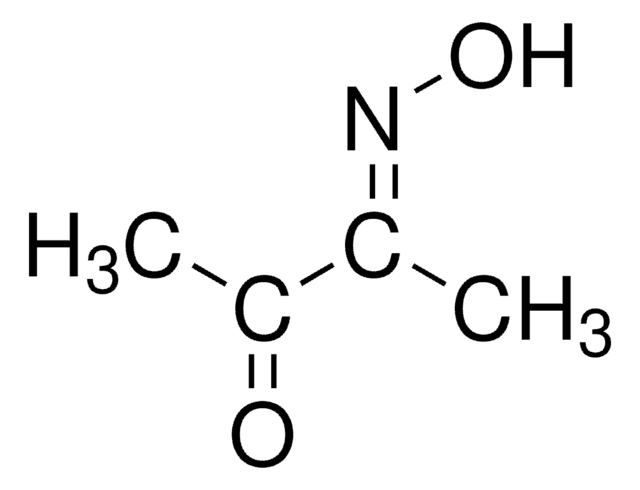
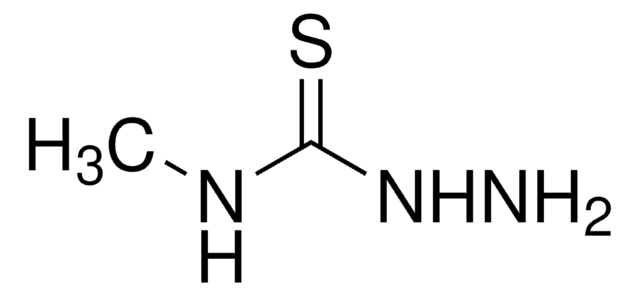
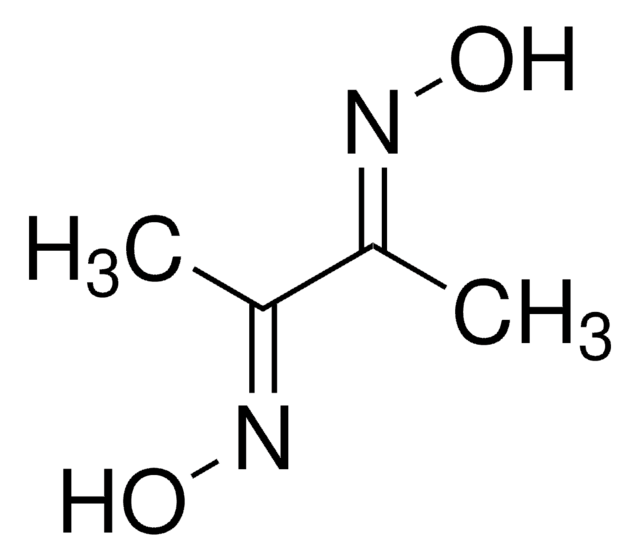
2,3-Butanedione monoxime
for spectrophotometric det. of urea, ≥99.0%
Sinônimo(s):
BDM, Biacetyl monoxime, Diacetyl monoxime
About This Item
Produtos recomendados
Nível de qualidade
Ensaio
≥98.0% (N)
≥99.0%
Formulário
solid
qualidade
for spectrophotometric det. of urea
técnica(s)
UV/Vis spectroscopy: suitable
resíduo de ignição
≤0.05% (as SO4)
p.e.
185-186 °C (lit.)
pf
75-76 °C
75-78 °C (lit.)
cadeia de caracteres SMILES
CC(=O)\C(C)=N\O
InChI
1S/C4H7NO2/c1-3(5-7)4(2)6/h7H,1-2H3/b5-3+
chave InChI
FSEUPUDHEBLWJY-HWKANZROSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Descrição geral
Aplicação
- A novel method to extend viability and functionality of living heart slices.: This research introduces a novel application of 2,3-Butanedione monoxime for prolonging the functional lifespan of cardiac tissue samples in experimental settings, offering insights into cardiac biology and potential therapeutic targets (Ross et al., 2023).
- Molecular Mechanisms of Deregulation of Muscle Contractility Caused by the R168H Mutation in TPM3 and Its Attenuation by Therapeutic Agents.: The study utilizes 2,3-Butanedione monoxime to investigate the molecular pathways affected by genetic mutations in muscle contractility, contributing to the understanding of muscle disorders and their management (Karpicheva et al., 2023).
- Generation of myocyte agonal Ca(2+) waves and contraction bands in perfused rat hearts following irreversible membrane permeabilisation.: Research employing 2,3-Butanedione monoxime investigates its role in inducing specific cellular events in cardiac cells under stress, highlighting its potential in studies of heart disease mechanisms and therapies (Morishita et al., 2023).
Nota de análise
0.5 g are completely soluble and give a clear solution in 10 mL water or also in 10 mL ethanol.
Sensitivity test:
0.05 mg urea in 3 mL water and 5 mL conc. HCl together with a 3% solution in water of diacetylmonoxime heated on a water bath for 10 minutes give a light yellow color.
Outras notas
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica