08198
Eleutheroside E
analytical standard
Sinônimo(s):
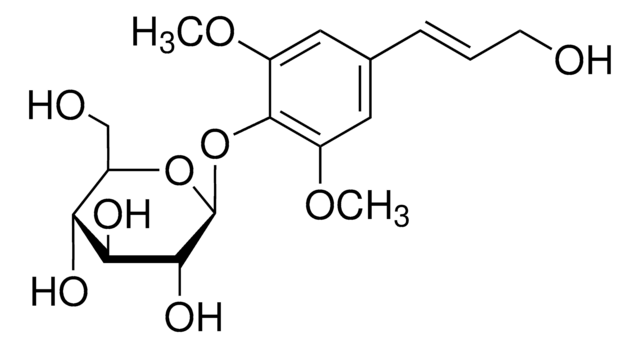
[(1R,3aR,4S,6aS)-Tetrahydro-1H,3H-furo[3,4-c]furan-1,4-diyl]bis(2,6-dimethoxy-4,1-phenylene) bis-β-D-glucopyranoside
About This Item
Produtos recomendados
grau
analytical standard
Nível de qualidade
Ensaio
≥98.0% (HPLC)
prazo de validade
limited shelf life, expiry date on the label
aplicação(ões)
food and beverages
Formato
neat
temperatura de armazenamento
2-8°C
cadeia de caracteres SMILES
COc1cc(cc(OC)c1O[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)[C@H]3OC[C@@H]4[C@@H]3CO[C@H]4c5cc(OC)c(O[C@@H]6O[C@H](CO)[C@@H](O)[C@H](O)[C@H]6O)c(OC)c5
InChI
1S/C34H46O18/c1-43-17-5-13(6-18(44-2)31(17)51-33-27(41)25(39)23(37)21(9-35)49-33)29-15-11-48-30(16(15)12-47-29)14-7-19(45-3)32(20(8-14)46-4)52-34-28(42)26(40)24(38)22(10-36)50-34/h5-8,15-16,21-30,33-42H,9-12H2,1-4H3/t15?,16?,21-,22+,23-,24+,25+,26-,27-,28+,29?,30?,33+,34-
chave InChI
FFDULTAFAQRACT-RGFZIUCCSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Aplicação
- Rat plasma and tissue by solid-phase extraction (SPE) followed by high-performance liquid chromatography (HPLC) and photodiode array detection (PDA).
- Acanthopanax senticosus by ionic liquids-ultrasound assisted extraction (ILUAE) followed by HPLC with ultraviolet (UV) detection.
- Eleutherococcus senticosus Maxim. by rapid resolution liquid chromatography (RRLC) equipped with multi-wavelength UV detector.
- Acanthopanax giraldii Harms by HPLC with diode array detector (DAD).
Embalagem
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica