MAB8661
Anti-Influenza B Antibody, nucleoprotein, clones B2, B4 Blend
ascites fluid, Chemicon®, from mouse
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Código UNSPSC:
12352203
eCl@ss:
32160702
NACRES:
NA.41
Produtos recomendados
fonte biológica
mouse
Nível de qualidade
forma do anticorpo
ascites fluid
tipo de produto de anticorpo
primary antibodies
clone
B2, monoclonal
B4, monoclonal
reatividade de espécies
human
fabricante/nome comercial
Chemicon®
técnica(s)
immunofluorescence: suitable
Isotipo
IgG
Condições de expedição
wet ice
Especificidade
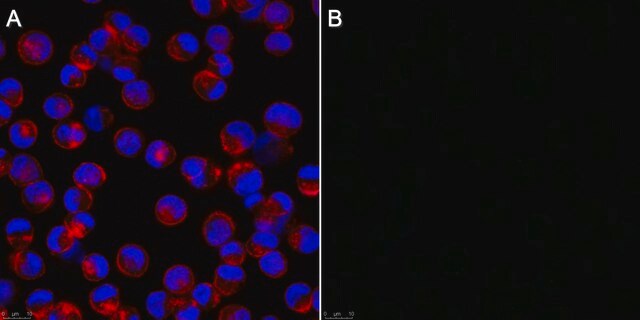
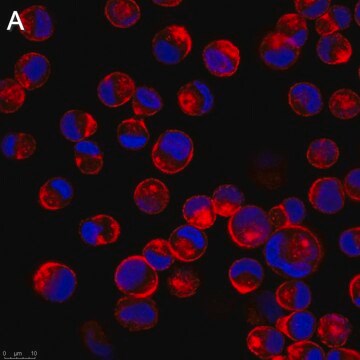
Recognizes all subtypes of Influenza B. Blend of two clones that recognize the nucleoprotein antigen.
No cross-reactivity to influenza A, parainfluenza 1, 2 or 3, adenovirus or RSV.
No cross-reactivity to influenza A, parainfluenza 1, 2 or 3, adenovirus or RSV.
Imunogênio
Influenza Blend.
Aplicação
Anti-Influenza B Antibody, nucleoprotein, clones B2, B4 Blend detects level of Influenza B & has been published & validated for use in IF.
Indirect immunofluorescence at 1:100.
Final working dilutions must be determined by end user.
Final working dilutions must be determined by end user.
Research Category
Infectious Diseases
Infectious Diseases
Research Sub Category
Infectious Diseases - Viral
Infectious Diseases - Viral
forma física
Ascites fluid with 0.1% sodium azide as a preservative.
Unpurified
Armazenamento e estabilidade
Maintain for 1 year at -20°C from date of shipment. Aliquot to avoid repeated freezing and thawing. For maximum recovery of product, centrifuge the original vial after thawing and prior to removing the cap.
Nota de análise
Control
Influenza Control Slides, Catalogue Number 5010-5
Influenza Control Slides, Catalogue Number 5010-5
Outras notas
Concentration: Please refer to the Certificate of Analysis for the lot-specific concentration.
Informações legais
CHEMICON is a registered trademark of Merck KGaA, Darmstadt, Germany
Exoneração de responsabilidade
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.
Não está encontrando o produto certo?
Experimente o nosso Ferramenta de seleção de produtos.
Código de classe de armazenamento
12 - Non Combustible Liquids
Classe de risco de água (WGK)
nwg
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Certificados de análise (COA)
Busque Certificados de análise (COA) digitando o Número do Lote do produto. Os números de lote e remessa podem ser encontrados no rótulo de um produto após a palavra “Lot” ou “Batch”.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Genetic variability of human metapneumovirus isolated from Chilean children, 2003-2004.
Carola Escobar,Vivian Luchsinger,Danielle Bruna de Oliveira,Edison Durigon et al.
Journal of Medical Virology null
Kevin R McCarthy et al.
Proceedings of the National Academy of Sciences of the United States of America, 118(23) (2021-06-03)
Immune memory of a first infection with influenza virus establishes a lasting imprint. Recall of that memory dominates the response to later infections or vaccinations by antigenically drifted strains. Early childhood immunization before infection may leave an imprint with different
Aaron G Schmidt et al.
Cell, 161(5), 1026-1034 (2015-05-12)
Vaccines for rapidly evolving pathogens will confer lasting immunity if they elicit antibodies recognizing conserved epitopes, such as a receptor-binding site (RBS). From characteristics of an influenza-virus RBS-directed antibody, we devised a signature motif to search for similar antibodies. We
Paulina Koszalka et al.
Antiviral research, 164, 91-96 (2019-02-17)
Baloxavir Marboxil (BXM) is an influenza polymerase inhibitor antiviral that binds to the endonuclease region in the PA subunit of influenza A and B viruses. To establish the baseline susceptibility of viruses circulating prior to licensure of BXM and to
Tina Schmidt et al.
European journal of immunology, 42(7), 1755-1766 (2012-05-16)
Antigen-specific antibodies are well characterized after vaccination with pandemic H1N1 or seasonal influenza vaccines. However, knowledge on cellular immunity toward pandemic H1N1 after vaccination and infection and cross-reactivities toward seasonal antigens is limited. Nineteen individuals were vaccinated with the pandemic
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica