8.51012
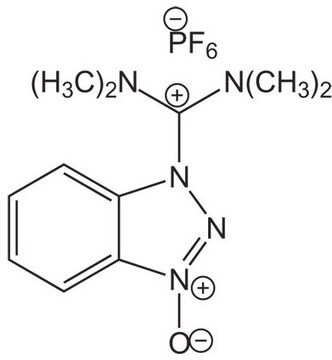
HCTU
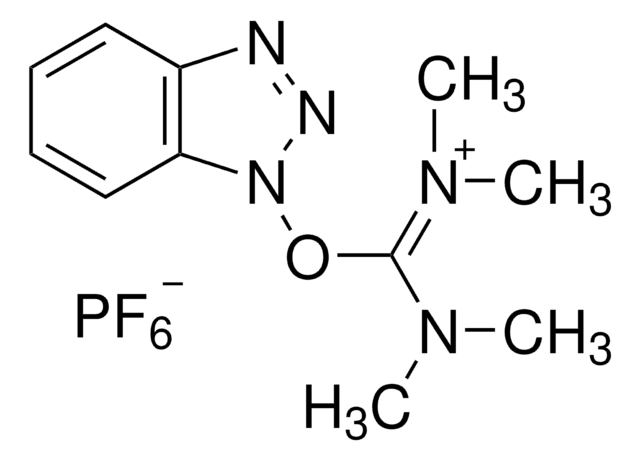
O-(1H-6-Chlorobenzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate Novabiochem®
Sinônimo(s):
2-(6-Chloro-1-H-benzotriazole-1-yl)-1,1,3,3-tetramethylaminium hexafluorophosphate, HCTU
About This Item
Produtos recomendados
Nível de qualidade
linha de produto
Novabiochem®
Ensaio
≥98% (HPLC)
Formulário
powder
adequação da reação
reaction type: Coupling Reactions
fabricante/nome comercial
Novabiochem®
pf
>185 °C
aplicação(ões)
peptide synthesis
temperatura de armazenamento
2-8°C
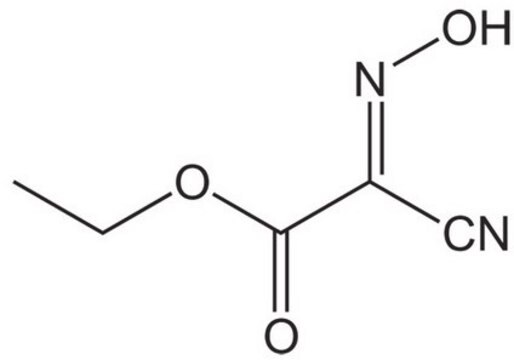
cadeia de caracteres SMILES
F[P-](F)(F)(F)(F)F.Clc1cc2[n](nnc2cc1)[O+]=C(N(C)C)N(C)C
InChI
1S/C11H15ClN5O.F6P/c1-15(2)11(16(3)4)18-17-10-7-8(12)5-6-9(10)13-14-17;1-7(2,3,4,5)6/h5-7H,1-4H3;/q+1;-1
chave InChI
ZHHGTMQHUWDEJF-UHFFFAOYSA-N
Descrição geral
Associated Protocols and Technical Articles
Guide to Selection of Coupling Reagents
Literature references
[1] O. Marder, et al. (2002) Chimica Oggi, 37.
[2] G. Sabatino, et al. in ′Peptides 2002, Proceedings of the 27th European Peptide Symposium′, E. Benedetti & C. Pedone (Eds), Naples, Edizioni Ziino, 2002, pp. 272.
[3] M. Gude & S. Barthélémy in ′Peptides 2002, Proceedings of the 27th European Peptide Symposium′, E. Benedetti & C. Pedone (Eds), Naples, Edizioni Ziino, 2002, pp. 122.
[4] G. Sabatino, et al. in ′Peptide Revolution: Genomics, Proteomics & Therapeutics, , Proc. 18th American Peptide Symposium′, M. Chrev & T. K. Sawyer (Eds), Cardiff, American Peptide Society, 2003, pp. 49.
Aplicação
- High-throughput parallel synthesis optimization of Glucagon-like Peptide 1 receptor agonists: Describes the use of HCTU as a reactive coupling reagent in peptide synthesis, highlighting its effectiveness in the production of GLP-1 agonists. (Ramos-Colón et al., 2018).
- An efficient synthesis of quinoxaline derivatives using HCTU as catalyst in DMF: Outlines a novel method for synthesizing quinoxaline and its derivatives using HCTU, highlighting its catalytic properties in facilitating efficient chemical reactions. (Sasane et al., 2023).
- An efficient one-pot conversion of carboxylic acids into benzimidazoles via an HBTU-promoted methodology: Describes the use of HCTU in a methodology to convert carboxylic acids into benzimidazoles, noting its role alongside other carbodiimide agents. (Barasa and Yoganathan, 2018).
Ligação
Nota de análise
Appearance of substance (visual): powder
Identity (IR): passes test
Assay (HPLC, area%): ≥ 98.0 % (a/a)
Solubility (1 mmole in 2 ml DMF): clearly soluble
Water (K. F.): ≤ 0.50 %
To see the solvent systems used for TLC of Novabiochem® products please click here.
Informações legais
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Skin Sens. 1A
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Certificados de análise (COA)
Busque Certificados de análise (COA) digitando o Número do Lote do produto. Os números de lote e remessa podem ser encontrados no rótulo de um produto após a palavra “Lot” ou “Batch”.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Artigos
Novabiochem® offers a large number of coupling reagents for in situ activation. In situ activating reagents are easy to use, fast reacting – even with sterically hindered amino acids, and their use is generally free of side reactions.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica