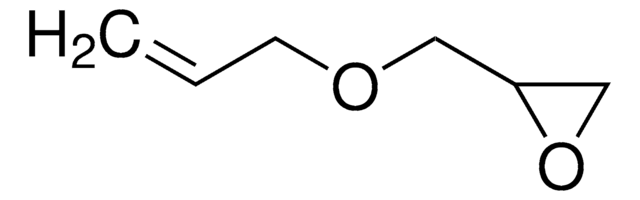
8.00609
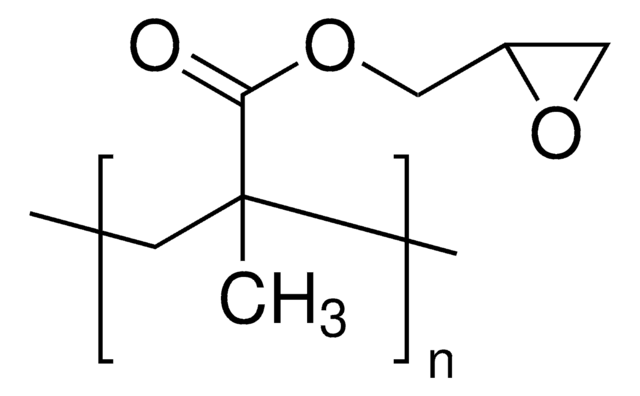
2,3-Epoxypropyl methacrylate
Technipur®, for synthesis
Sinônimo(s):
Glycidyl methacrylate, 2-((Methacryloxy)methyl)oxirane, 2,3-Epoxypropyl methacrylate, Methacrylic acid 2,3-epoxypropyl ester
About This Item
Produtos recomendados
grau
for synthesis
synthesis grade
Nível de qualidade
pressão de vapor
4.2 hPa ( 25 °C)
índice de refração
n20/D 1.449 (lit.)
p.e.
189 °C (lit.)
pf
<-60 °C
densidade
1.042 g/mL at 25 °C (lit.)
1.07 g/cm3 at 25 °C
cadeia de caracteres SMILES
CC(=C)C(=O)OCC1CO1
InChI
1S/C7H10O3/c1-5(2)7(8)10-4-6-3-9-6/h6H,1,3-4H2,2H3
chave InChI
VOZRXNHHFUQHIL-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Aplicação
It is also used to prepare poly(n-butyl methacrylate-co-glycidyl methacrylate) copolymers, which find applications in adhesives, surface coatings, and electrical devices in various polymeric industries. The presence of epoxy groups and flexible butyl groups in epoxy resin-modified copolymers increases its mechanical properties, impact strength, and fracture elongation.
Informações legais
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 3 Dermal - Acute Tox. 4 Oral - Carc. 1B - Eye Dam. 1 - Muta. 2 - Repr. 1B - Skin Corr. 1C - Skin Sens. 1 - STOT RE 1 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
6.1C - Combustible, acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
168.8 °F - closed cup
Ponto de fulgor (°C)
76 °C - closed cup
Certificados de análise (COA)
Busque Certificados de análise (COA) digitando o Número do Lote do produto. Os números de lote e remessa podem ser encontrados no rótulo de um produto após a palavra “Lot” ou “Batch”.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica