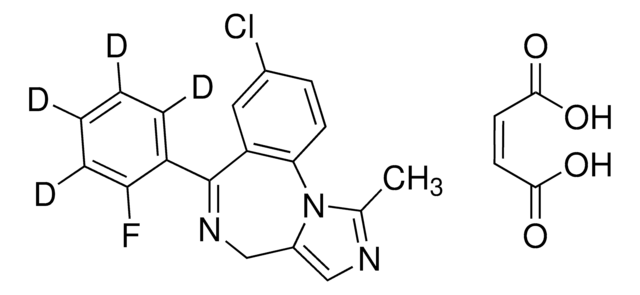
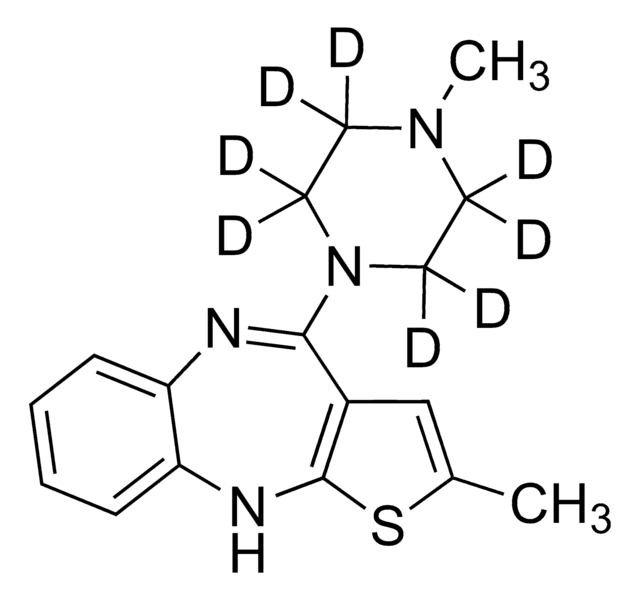
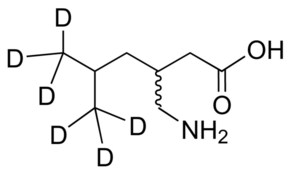
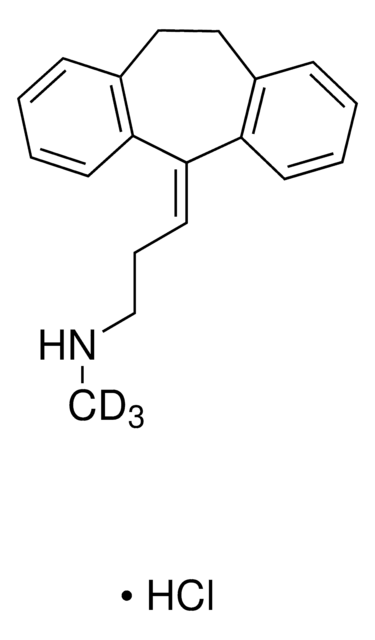
M-144
MDAI hydrochloride solution
100 μg/mL in acetonitrile: water (9:1) with 5% 1 M HCl (as free base), ampule of 1 mL, certified reference material, Cerilliant®
About This Item
Produtos recomendados
grau
certified reference material
Formulário
liquid
Características
Snap-N-Spike®/Snap-N-Shoot®
embalagem
ampule of 1 mL
fabricante/nome comercial
Cerilliant®
drug control
Narcotic Licence Schedule E (Switzerland)
concentração
100 μg/mL in acetonitrile: water (9:1) with 5% 1 M HCl (as free base)
técnica(s)
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
aplicação(ões)
forensics and toxicology
Formato
single component solution
temperatura de armazenamento
−70°C
cadeia de caracteres SMILES
Cl.NC1Cc2cc3OCOc3cc2C1
InChI
1S/C10H11NO2.ClH/c11-8-1-6-3-9-10(13-5-12-9)4-7(6)2-8;/h3-4,8H,1-2,5,11H2;1H
chave InChI
DEZYWEZDXRXACY-UHFFFAOYSA-N
Descrição geral
Informações legais
produto relacionado
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Liq. 2
Código de classe de armazenamento
3 - Flammable liquids
Classe de risco de água (WGK)
WGK 2
Ponto de fulgor (°F)
55.4 °F - closed cup
Ponto de fulgor (°C)
13 °C - closed cup
Escolha uma das versões mais recentes:
Certificados de análise (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documentos section.
Se precisar de ajuda, entre em contato Atendimento ao cliente
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica