H-059
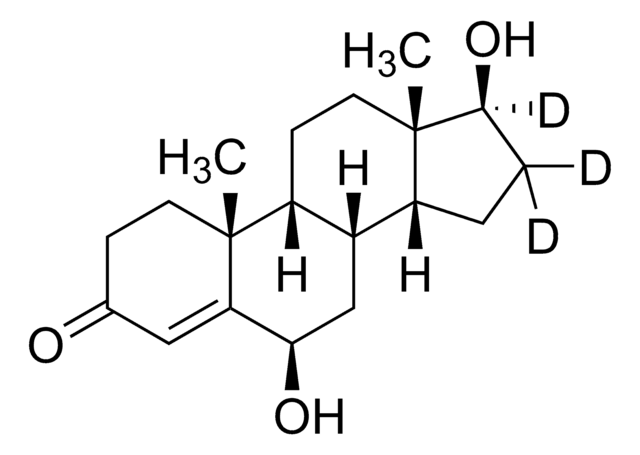
6β-Hydroxytestosterone solution
100 μg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®
About This Item
Produtos recomendados
grau
certified reference material
Nível de qualidade
Formulário
liquid
Características
Snap-N-Spike®/Snap-N-Shoot®
embalagem
ampule of 1 mL
fabricante/nome comercial
Cerilliant®
concentração
100 μg/mL in methanol
técnica(s)
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
aplicação(ões)
clinical testing
Formato
single component solution
temperatura de armazenamento
−20°C
cadeia de caracteres SMILES
C[C@]12CC[C@H]3[C@@H](C[C@@H](O)C4=CC(=O)CC[C@]34C)[C@@H]1CC[C@@H]2O
InChI
1S/C19H28O3/c1-18-7-5-11(20)9-15(18)16(21)10-12-13-3-4-17(22)19(13,2)8-6-14(12)18/h9,12-14,16-17,21-22H,3-8,10H2,1-2H3/t12-,13-,14-,16+,17-,18+,19-/m0/s1
chave InChI
XSEGWEUVSZRCBC-ZVBLRVHNSA-N
Descrição geral
Informações legais
produto relacionado
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
Órgãos-alvo
Eyes
Código de classe de armazenamento
3 - Flammable liquids
Classe de risco de água (WGK)
WGK 2
Ponto de fulgor (°F)
49.5 °F - closed cup
Ponto de fulgor (°C)
9.7 °C - closed cup
Escolha uma das versões mais recentes:
Certificados de análise (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documentos section.
Se precisar de ajuda, entre em contato Atendimento ao cliente
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Artigos
Separation of Testosterone solution, 1.0 mg/mL in acetonitrile, ampule of 1 mL, certified reference material; 17α-Methyltestosterone solution, 1.0 mg/mL in 1,2-dimethoxyethane, ampule of 1 mL, certified reference material; 5α-Dihydrotestosterone (DHT) solution, 1.0 mg/mL in methanol, ampule of 1 mL, certified reference material; 6β-Hydroxytestosterone solution, 100 μg/mL in methanol, ampule of 1 mL, certified reference material
Global Trade Item Number
| SKU | GTIN |
|---|---|
| H-059-1ML | 4061833662007 |
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica