V209
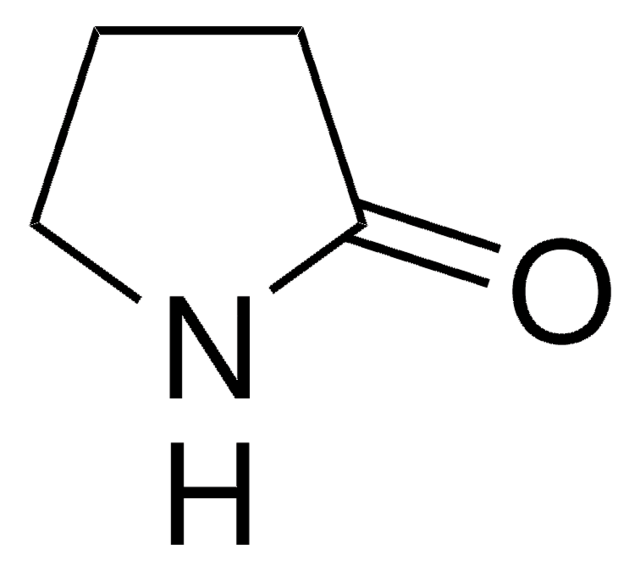
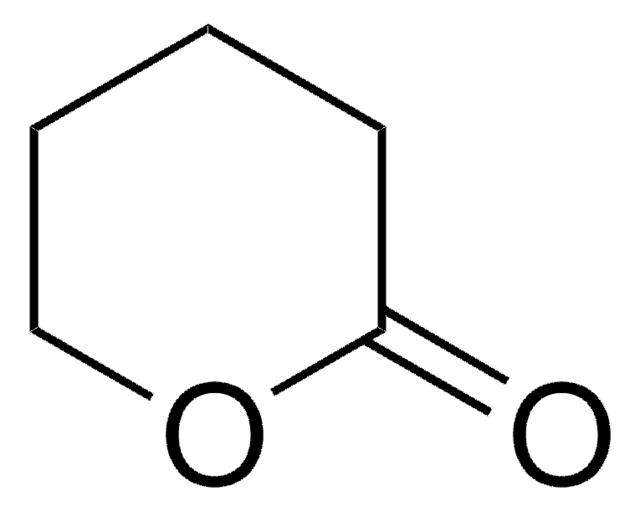
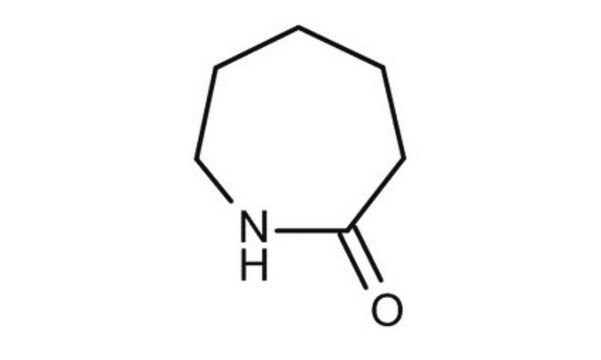
δ-Valerolactam
98%
Sinônimo(s):
delta-Valerolactam, 2-Piperidone
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C5H9NO
Número CAS:
Peso molecular:
99.13
Beilstein:
106434
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
98%
p.e.
256 °C (lit.)
81-82 °C/0.1 mmHg (lit.)
pf
38-40 °C (lit.)
cadeia de caracteres SMILES
O=C1CCCCN1
InChI
1S/C5H9NO/c7-5-3-1-2-4-6-5/h1-4H2,(H,6,7)
chave InChI
XUWHAWMETYGRKB-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
235.4 °F - closed cup
Ponto de fulgor (°C)
113 °C - closed cup
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Ahmed Mahjoub et al.
Chemphyschem : a European journal of chemical physics and physical chemistry, 12(10), 1822-1832 (2011-05-28)
We studied the single-photon ionization of gas-phase δ-valerolactam (piperidin-2-one) and of its dimer using vacuum-ultraviolet (VUV) synchrotron radiation coupled to a velocity map imaging electron/ion coincidence spectrometer. The slow photoelectron spectrum (SPES) of the monomer is dominated by the vibrational
Metabolic engineering of Escherichia coli for the production of four-, five- and six-carbon lactams.
Tong Un Chae et al.
Metabolic engineering, 41, 82-91 (2017-04-10)
Microbial production of chemicals and materials from renewable sources is becoming increasingly important for sustainable chemical industry. Here, we report construction of a new and efficient platform metabolic pathway for the production of four-carbon (butyrolactam), five-carbon (valerolactam) and six-carbon (caprolactam)
Moitrayee Mukherjee et al.
The journal of physical chemistry. A, 116(40), 9888-9896 (2012-09-19)
A comparative analysis for relative stability between normal and tautomeric forms in the excited electronic states of 7-azaindole···δ-valerolactam 1:1 complex and 7-azaindole homodimer has been presented. The tautomeric configuration of the complex is estimated to be ~6 kcal/mol more stable
Asymmetric synthesis of gamma-keto-delta-lactam derivatives: application to the synthesis of a conformationally constrained surrogate of Ala-Ser dipeptide.
S D Koulocheri et al.
The Journal of organic chemistry, 66(23), 7915-7918 (2001-11-10)
S Gordon et al.
Farmaco (Societa chimica italiana : 1989), 52(10), 603-608 (1998-05-15)
The synthesis of a series of 2-amino-4-hydroxy-delta-valerolactam derivatives is described (compounds 4 to 10). These compounds showed a high anthelmintic in vitro activity against the Nippostrongylus brasiliensis model.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica