P10801
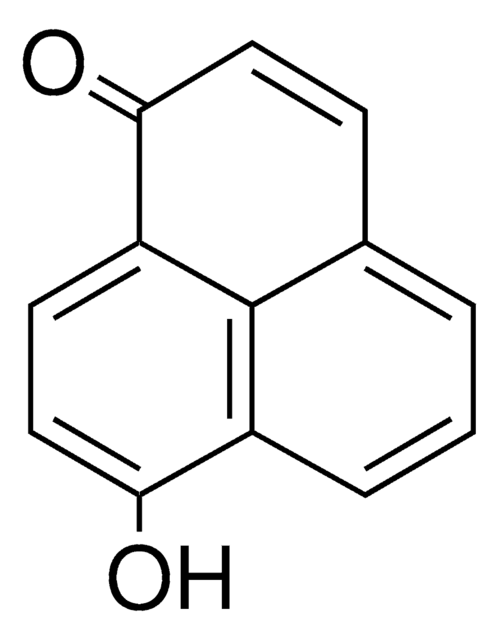
Perinaphthenone
97%
Sinônimo(s):
1H-Benzonaphthen-1-one, 7-Perinaphthenone, Phenalenone, Phenalone
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C13H8O
Número CAS:
Peso molecular:
180.20
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
97%
Formulário
powder
pf
153-156 °C (lit.)
cadeia de caracteres SMILES
O=C1C=Cc2cccc3cccc1c23
InChI
1S/C13H8O/c14-12-8-7-10-4-1-3-9-5-2-6-11(12)13(9)10/h1-8H
chave InChI
WWBGWPHHLRSTFI-UHFFFAOYSA-N
Aplicação
Perinaphthenone can be used:
- In the Weitz-Scheffer epoxidation to produce the corresponding epoxides in the presence of gem-dihydroperoxide as the stoichiometric oxidant.
- As a photosensitizer in the photodegradation of tebuconazole.
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Mahmoud Fahmi Elsebai et al.
Organic & biomolecular chemistry, 9(3), 802-808 (2010-11-26)
The marine-derived fungus Coniothyrium cereale was isolated from the green alga Enteromorpha sp. and found to produce the new phenalenone derivatives 1-7 as well as the known compounds lactone 8, (-) sclerodin (9), lamellicolic anhydride (10), (-) scleroderolide (11), and
Christopher M Whidbey et al.
Water research, 46(16), 5287-5296 (2012-08-11)
Steroid estrogens are endocrine disrupting contaminants frequently detected in natural waters. Because these estrogens can elicit significant biological responses in aquatic organisms, it is important to study their rates and pathways of degradation in natural waters and to identify whether
Zhiyong Guo et al.
Magnetic resonance in chemistry : MRC, 45(5), 439-441 (2007-03-21)
The complete (1)H and (13)C NMR assignments are reported for the novel natural product Bacillosporin D together with the known compound Bacillosporin C. These compounds containing seven rings were isolated from the mangrove endophytic fungus SBE-14 from the South China
Cristina Flors et al.
Accounts of chemical research, 39(5), 293-300 (2006-05-17)
Plants defend themselves from pathogen infections or mechanical injury by a number of mechanisms, including the induced biosynthesis of antimicrobial secondary metabolites. These compounds, termed phytoalexins, represent a very economical way to counteract hazard, because the carbon and energy resources
William Hidalgo et al.
Journal of agricultural and food chemistry, 57(16), 7417-7421 (2009-07-28)
The levels of native fungitoxic perinaphthenone phytoalexins in susceptible Musa varieties (banana), which are commercially grown in large plantations, are too low to provide plants with long-lasting protection against highly pathogenic fungi. Novel strategies for plant protection are necessary to
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica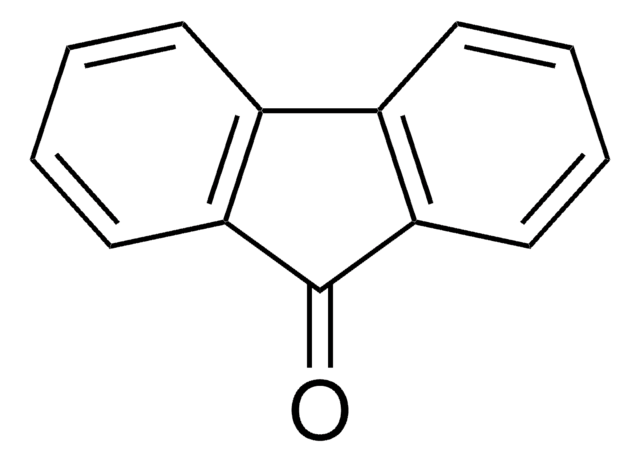

![Cyclopenta[d,e,f]phenanthrene 97%](/deepweb/assets/sigmaaldrich/product/structures/107/640/eed40ce0-e715-4438-9cb3-dc3dc13dcb9b/640/eed40ce0-e715-4438-9cb3-dc3dc13dcb9b.png)