I17451
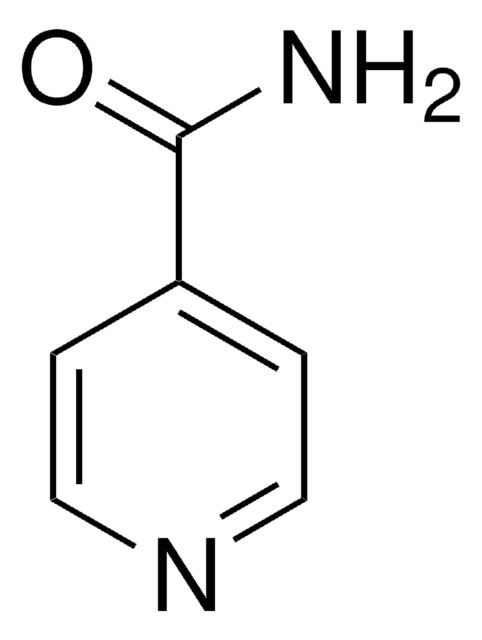
Isonicotinamide
ReagentPlus®, 99%
Sinônimo(s):
Pyridine-4-carboxylic acid amide
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C6H6N2O
Número CAS:
Peso molecular:
122.12
Beilstein:
2173
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
linha de produto
ReagentPlus®
Ensaio
99%
pf
155-157 °C (lit.)
cadeia de caracteres SMILES
NC(=O)c1ccncc1
InChI
1S/C6H6N2O/c7-6(9)5-1-3-8-4-2-5/h1-4H,(H2,7,9)
chave InChI
VFQXVTODMYMSMJ-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Aplicação
Isonicotinamide (pyridine-4-carboxamide) can be used as a heterocyclic building block to synthesize:
It can also be used as a co-former with active pharmaceutical ingredients (APIs) to prepare co-crystals.
- 4-oxo-1,3-thiazinan-3-yl isonicotinamide derivatives as potential anti-tubercular agents.
- Organotin(IV) complexes of isonicotinamide via synthesis of phosphoramidate ligands for various biological activity studies.
- Bis-pyridinium isonicotinamide derivatives of 2-(hydroxyimino)-N-(pyridin-3-yl)acetamide as potent reactivators sarin.
It can also be used as a co-former with active pharmaceutical ingredients (APIs) to prepare co-crystals.
Informações legais
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Gareth Arnott et al.
Organic letters, 10(14), 3089-3092 (2008-06-17)
Treatment of N-arylisonicotinamides with trifluoromethanesulfonic anhydride triggers intramolecular nucleophilic attack of the aryl ring on the 4-position of the pyridinium intermediate. The products are spirocyclic dihydropyridines which can be converted to valuable spirocyclic piperidines related to biologically active molecules such
Rodrigo A de Souza et al.
European journal of medicinal chemistry, 45(11), 4863-4868 (2010-08-21)
Complexes of the type trans-[PdX(2)(isn)(2)] {X = Cl (1), N(3) (2), SCN (3), NCO (4); isn = isonicotinamide} were synthesized and evaluated for in vitro antimycobacterial and antitumor activities. The coordination mode of the isonicotinamide and the pseudohalide ligands was
Jemma Senczyszyn et al.
Organic letters, 15(8), 1922-1925 (2013-04-04)
On treatment with acylating or sulfonylating agents, N-alkenyl pyridine carboxamides (N-pyridinecarbonyl enamines) undergo a dearomatizing cyclization initiated by pyridine acylation and followed by intramolecular trapping of the resulting pyridinium cation. The products are spirocyclic dihydropyridines which may be further elaborated
Sarah Boyd et al.
Journal of pharmaceutical sciences, 99(9), 3779-3786 (2010-07-29)
The solubility and crystal growth of the 1:1 cocrystal between benzoic acid and isonicotinamide from 95% ethanol was studied through the creation of a ternary phase diagram at differing temperatures and turbidity measurements. From the solubility measurements thermodynamic properties of
Shaun R Stauffer et al.
Bioorganic & medicinal chemistry letters, 17(6), 1788-1792 (2007-01-30)
A series of low-molecular weight 2,6-diamino-isonicotinamide BACE-1 inhibitors containing an amine transition-state isostere were synthesized and shown to be highly potent in both enzymatic and cell-based assays. These inhibitors contain a trans-S,S-methyl cyclopropane P(3) which bind BACE-1 in a 10s-loop
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica