A47052
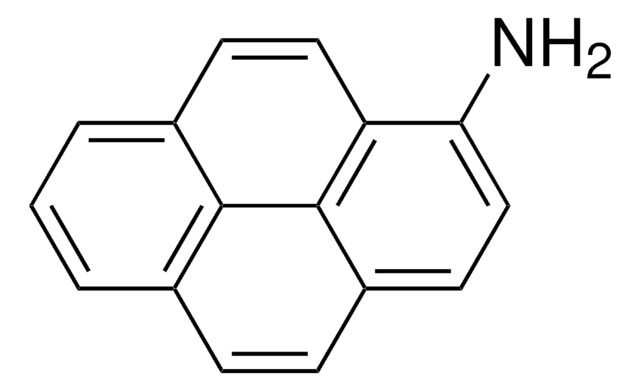
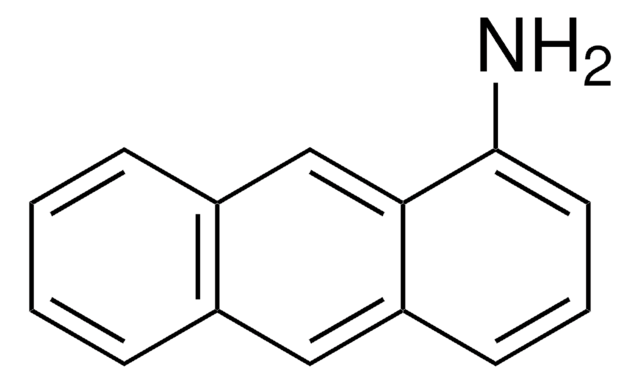
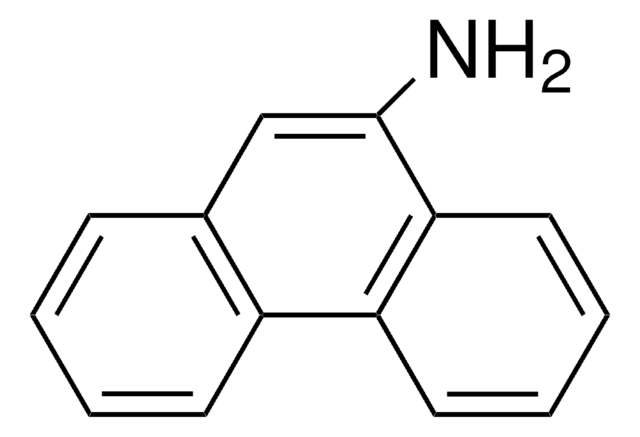
6-Aminochrysene
95%
Sinônimo(s):
6-Chrysenamine
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C18H13N
Número CAS:
Peso molecular:
243.30
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Ensaio
95%
Formulário
solid
pf
209-211 °C (lit.)
cadeia de caracteres SMILES
Nc1cc2c3ccccc3ccc2c4ccccc14
InChI
1S/C18H13N/c19-18-11-17-13-6-2-1-5-12(13)9-10-15(17)14-7-3-4-8-16(14)18/h1-11H,19H2
chave InChI
KIVUHCNVDWYUNP-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Aplicação
Produces tumors in mice.
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Oral
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
B G Lake et al.
Toxicology and applied pharmacology, 138(2), 231-241 (1996-06-01)
Precision-cut liver slices were prepared from male Sprague-Dawley rats (pretreated with or without Aroclor 1254), male Dunkin-Hartley guinea pigs, male cynomolgus monkeys, and humans. Liver slices were cultured for 24 hr using a dynamic organ culture system in medium containing
H Yamazaki et al.
Carcinogenesis, 15(3), 465-470 (1994-03-01)
In order to address the hypothesis that 6-aminochrysene (6-AC) is converted to genotoxic products by cytochrome P450 enzymes via two activation pathways (N-hydroxylation and epoxidation), the activation of 6-AC and trans-1,2-dihydro-1,2-dihydroxy-6-aminochrysene (6-AC-diol) to genotoxic metabolites was examined in rat and
K B Delclos et al.
Carcinogenesis, 8(11), 1703-1709 (1987-11-01)
Since 6-nitrochrysene and 6-aminochrysene have shown activity in carcinogenicity bioassays, we have begun an investigation of their metabolic activation pathways and the nature of the carcinogen-DNA adducts that may be formed. N-Hydroxy-6-aminochrysene (N-hydroxy-AC), a candidate proximate or ultimate carcinogen and
K B Delclos et al.
IARC scientific publications, (124)(124), 79-86 (1993-01-01)
Carcinogenic arylamines and nitroaromatic hydrocarbons are chemicals that present occupational health hazards and share pathways of metabolic activation. The 32P-postlabelled DNA adducts formed in Chinese hamster ovary (CHO) cells treated with metabolites from two pathways that are common to the
M Mimura et al.
Drug metabolism and disposition: the biological fate of chemicals, 21(6), 1048-1056 (1993-11-01)
A cytochrome P-450 (P-450) enzyme of the CYP2B subfamily was partially purified from human liver microsomes and characterized with respect to immunochemical properties, N-terminal amino acid sequence, and catalytic activities toward typical P-450 substrates. P-450 enzymes were monitored in chromatographic
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica