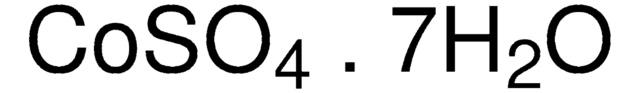939331
Nickel(II) sulfate hexahydrate
≥99.9% trace metals basis
Sinônimo(s):
Nickelous Sulfate, Hexahydrate, Battery grade nickel sulfate, Nickel monosulfate hexahydrate, Nickel sulphate hexahydrate
About This Item
Produtos recomendados
grau
for analytical purposes
Nível de qualidade
tipo
(High purity Salts)
Ensaio
≥99.9% trace metals basis
98-102% (EDTA, complexometric)
Formulário
powder or crystals
Impurezas
≤1000 ppm trace metals basis
cor
faint blue to dark blue-green
pH
4.3-4.7 (20 °C, 100 g/L in water)
solubilidade
water: soluble
densidade
2.07 g/cm3 at 20 °C
traços de ânion
chloride (Cl-): ≤20 ppm
traços de cátion
Al: <50 ppm
Ca: <50 ppm
Co: <50 ppm
Cu: <50 ppm
Fe: <50 ppm
K: <50 ppm
Mg: <50 ppm
Na: <50 ppm
Pb: <50 ppm
Zn: <50 ppm
cadeia de caracteres SMILES
[Ni+2].[S](=O)(=O)([O-])[O-].O.O.O.O.O.O
InChI
1S/Ni.H2O4S.6H2O/c;1-5(2,3)4;;;;;;/h;(H2,1,2,3,4);6*1H2/q+2;;;;;;;/p-2
chave InChI
RRIWRJBSCGCBID-UHFFFAOYSA-L
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Aplicação
Therefore, Nickel(II) sulfate hexahydrate has been widely used as a key component for the synthesis of Cathode for Lithium-ion batteries. - Spherical NCM 622 and Li[Ni0.9Co0.05Mn0.05]O2 (NCM 900505) were synthesized via a co-precipitation method using Nickel(II) sulfate hexahydrate. In order to achieve the desired energy density, it is necessary to maximize both the nickel content in the cathode and the cutoff voltage. [] - to synthesis single-crystal, Ni-rich NCM and polycrystalline NCM cathodes with various Ni content by coprecipitation method . These Ni-rich layered cathodes like NCM, NCA, and NCMA ([Ni1–x–yCox(Mn and/or Al)y]O2) are the top choices for powering upcoming electric vehicles. It is found that polycrystalline NCM cathodes are prone to intergranular microcracking during cycling, single crystal NCM cathodes demonstrate resilience against mechanical fracture, even under highly charged conditions or repeated cycles. Due to limited lithium-ion diffusion pathways, the electrochemical performance of single crystal -NCM cathodes, particularly in terms of capacity and cycling stability, is lower compared to that of polycrystalline-NCM cathodes. The difference in the electrochemical performance of single crystal -NCM and polycrystalline-NCM cathodes grows as the Ni fraction increases. In addition, Nickel(II) sulfate hexahydrate is widely used for electroplating for producing metallic coatings. Nickel(II) sulfate hexahydrate can also be used as a catalyst in: -Pt50Ni50 catalysts supported on MCM-41 were produced using wet co-impregnation. These catalysts were then employed for hydrogenation reactions of benzene in gas phase. The morphology of the metal phase within the catalysts has a notable impact on the conversion of benzene to cyclohexane. Factors like reduction temperature, NaBH4 concentration, and reduction medium influence the particle morphology. []
Características e benefícios
- Water soluble
- Medium purity (99.9%)
- Low trace metals in ppm level
- Cost effective Suitable for battery applications
- Recycled catalyst
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 1A Inhalation - Muta. 2 - Repr. 1B - Resp. Sens. 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT RE 1 Inhalation
Órgãos-alvo
Respiratory Tract
Código de classe de armazenamento
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Certificados de análise (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documentos section.
Se precisar de ajuda, entre em contato Atendimento ao cliente
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica