913006
IEICO
≥99%
Sinônimo(s):
2,2′-((2Z,2′Z)-((5,5′-bis(4,4,9,9-tetrakis(4-hexylphenyl)-4,9-dihydro-s-indaceno[1,2-b:5,6-b′]dithiophene-2,7-diyl)bis(4-((2- ethylhexyl)oxy)thiophene-5,2-diyl))bis(methanylylidene))bis-(3-oxo-2,3-dihydro-1H-indene-2,1-diylidene))dimalononitrile, 2,2′-[[4,4,9,9-tetrakis(4-hexylphenyl)-4,9-dihydro-s-indaceno[1,2-b:5,6-b′]dithiophene-2,7-diyl]bis[[4-[(2-ethylhexyl)oxy]-5,2-thiophenediyl]-(Z)-methylidyne(3-oxo-1H-indene-2,1(3H)-diylidene)]]bis-propanedinitrile
About This Item
Produtos recomendados
descrição
Band gap: Eg=1.34 eV
Nível de qualidade
Ensaio
≥99%
Formulário
solid
cor
dark
Energia orbital
HOMO -5.32 eV
LUMO -3.95 eV
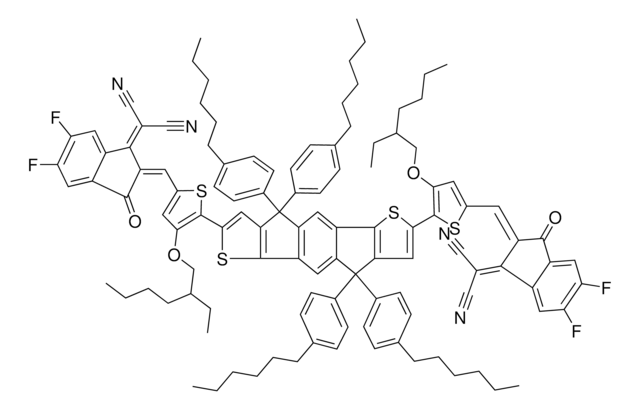
cadeia de caracteres SMILES
[s]1c(c(cc1\C=C%14/C(=O)c%15c(cccc%15)C/%14=C(C#N)C#N)OCC(CCCC)CC)c2[s]c3c(c2)C(c6c3cc7c(c6)c8[s]c(cc8C7(c%13ccc(cc%13)CCCCCC)c%12ccc(cc%12)CCCCCC)c9[s]c(cc9OCC(CCCC)CC)\C=C%10/C(=O)c%11c(cccc%11)C/%10=C(C#N)C#N)(c5ccc(cc5)CCCCCC)c4ccc(cc4)CCCCCC
Categorias relacionadas
Aplicação
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Certificados de análise (COA)
Lamentamos, não temos COA para este produto disponíveis online no momento.
Se precisar de ajuda, entre em contato Atendimento ao cliente
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica