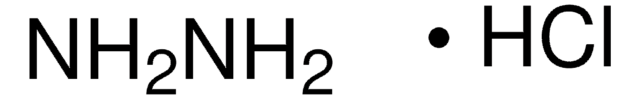751855
Hydrazine solution
1 M in acetonitrile
Sinônimo(s):
Hydrazine, Nitrogen hydride
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula linear:
NH2NH2
Número CAS:
Peso molecular:
32.05
Beilstein:
878137
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
forma
liquid
Nível de qualidade
concentração
1 M in acetonitrile
índice de refração
n20/D 1.348
densidade
0.779 g/mL at 25 °C
grupo funcional
hydrazine
cadeia de caracteres SMILES
NN
InChI
1S/H4N2/c1-2/h1-2H2
chave InChI
OAKJQQAXSVQMHS-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Hydrazine (N2H4) solution is a common reducing agent and a versatile reagent in organic synthesis. It can reduce a variety of functional groups like ketones, aldehydes, imines, nitro compounds, azides & nitrates to their corresponding amines. It also serves as a source of nitrogen in the synthesis of heterocyclic compounds, such as pyrazoles and pyrazolines.
Aplicação
Hydrazine solution (in acetonitrile) can be used as a reducing source for the reduction of carbon-carbon multiple bonds in alkenes, alkynes, and α,β-unsaturated esters using metal-organic frameworks (MOFs) as heterogeneous catalysts. It is also used in the synthesis of polysubstituted 2-aminoimidazoles from 2-aminopyrimidines and α-bromocarbonyl compounds.
produto relacionado
Nº do produto
Descrição
Preços
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 3 Inhalation - Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Aquatic Chronic 2 - Carc. 1B - Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - Skin Sens. 1
Código de classe de armazenamento
3 - Flammable liquids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
41.0 °F
Ponto de fulgor (°C)
5 °C
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
D P Elder et al.
Journal of pharmaceutical and biomedical analysis, 54(5), 900-910 (2010-12-15)
This is the latest of a series of reviews focused on the analysis of genotoxic impurities. This review summarises the analytical approaches reported in the literature relating to hydrazine, hydrazines, hydrazides and hydrazones. It is intended to provide guidance for
Min Yuan et al.
Dalton transactions (Cambridge, England : 2003), (31)(31), 6078-6088 (2010-05-08)
A combination of unique solvent properties of hydrazine enables the direct dissolution of a range of metal chalcogenides at ambient temperature, rendering this an extraordinarily simple and soft synthetic approach to prepare new metal chalcogenide-based materials. The extended metal chalcogenide
M Vogel et al.
Fresenius' journal of analytical chemistry, 366(8), 781-791 (2001-03-03)
Hydrazine reagents are a well-known group of derivatizing agents for the determination of aldehydes and ketones in liquid and gaseous samples. Within this article, the most important hydrazine reagents are critically summarized, and their major applications in different fields, including
Nilay Hazari
Chemical Society reviews, 39(11), 4044-4056 (2010-06-24)
One of the most challenging problems in small molecule activation is the development of a homogeneous catalyst for converting dinitrogen into ammonia at ambient temperatures and atmospheric pressure. A catalytic cycle based on molybdenum that converts dinitrogen into ammonia has
Tino Wilson Sanchez et al.
Bioorganic & medicinal chemistry, 21(4), 957-963 (2013-01-12)
Human lens epithelium-derived growth factor (LEDGF)/p75 plays an important role in the HIV life cycle by stimulating integrase (IN)-led viral DNA integration into cellular chromosomes. Mechanistic studies show the majority of IN inhibitors chelate magnesium ions in the catalytic active
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica