727075
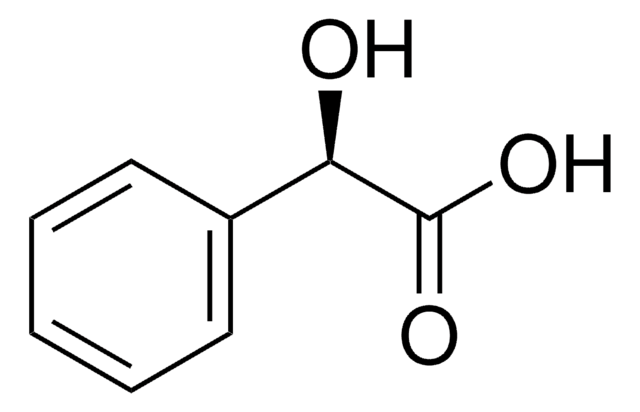
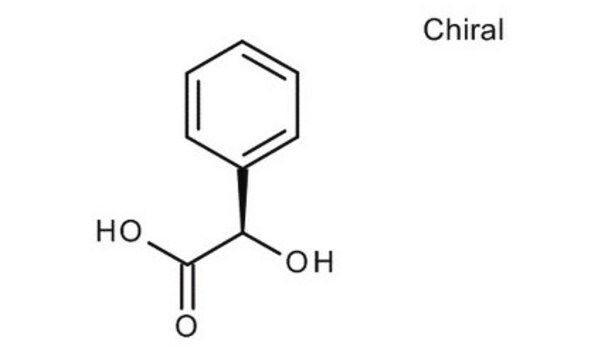
(R)-(−)-Mandelic acid
ChiPros®, produced by BASF, 99%
Sinônimo(s):
(R)-α-Hydroxyphenylacetic acid
About This Item
Produtos recomendados
grau
produced by BASF
Nível de qualidade
Ensaio
≥98.5%
99%
Formulário
crystals
pureza óptica
enantiomeric excess: ≥98.5%
pf
131-133 °C (lit.)
grupo funcional
carboxylic acid
hydroxyl
phenyl
cadeia de caracteres SMILES
O[C@@H](C(O)=O)c1ccccc1
InChI
1S/C8H8O3/c9-7(8(10)11)6-4-2-1-3-5-6/h1-5,7,9H,(H,10,11)/t7-/m1/s1
chave InChI
IWYDHOAUDWTVEP-SSDOTTSWSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Aplicação
- As a chiral acid in the separation of diastereomeric salts for the synthesis of chiral pyridoindole based building block.
- In the study of chiral acid selectivity of poly(3,4-ethylenedioxythiophene) (PEDOT) based chiral conducting polymers.
Informações legais
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Dam. 1
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 1
Ponto de fulgor (°F)
>374.0 °F
Ponto de fulgor (°C)
> 190 °C
Escolha uma das versões mais recentes:
Certificados de análise (COA)
Não está vendo a versão correta?
Se precisar de uma versão específica, você pode procurar um certificado específico pelo número do lote ou da remessa.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica