675792
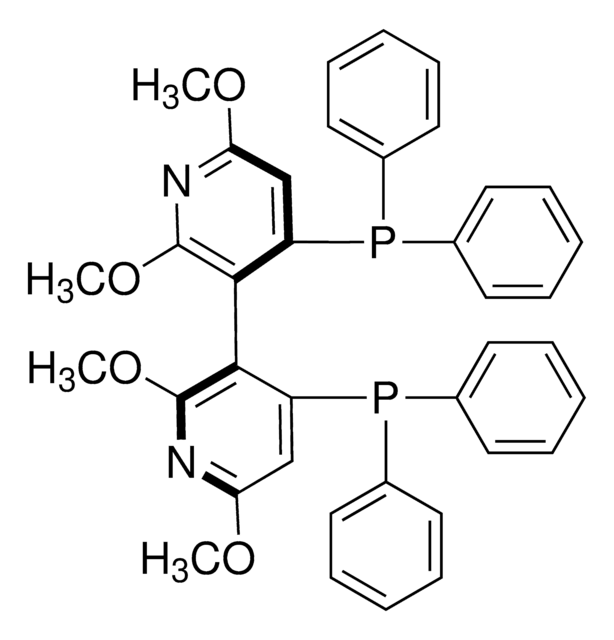
(S)-(−)-2,2′,6,6′-Tetramethoxy-4,4′-bis(diphenylphosphino)-3,3′-bipyridine
97%
Sinônimo(s):
(S)-P-Phos
About This Item
Produtos recomendados
Ensaio
97%
atividade óptica
[α]20/D -98°, c = 1 in chloroform
pf
261-265 °C
grupo funcional
phosphine
cadeia de caracteres SMILES
COc1cc(P(c2ccccc2)c3ccccc3)c(c(OC)n1)-c4c(OC)nc(OC)cc4P(c5ccccc5)c6ccccc6
InChI
1S/C38H34N2O4P2/c1-41-33-25-31(45(27-17-9-5-10-18-27)28-19-11-6-12-20-28)35(37(39-33)43-3)36-32(26-34(42-2)40-38(36)44-4)46(29-21-13-7-14-22-29)30-23-15-8-16-24-30/h5-26H,1-4H3
chave InChI
JZOSBBLJKXSBBN-UHFFFAOYSA-N
Categorias relacionadas
Aplicação
- In the asymmetic hydrogenation reactions.
- For the preparation of chiral ketone functionalized polymers by copolymerization reaction.
- To synthesize chiral alkynes by asymmetric hydroalkynylation of nonpolar alkenes or norbornadienes using iridium catalyst.
- In the selective allylic alkylation of indoles using palladium catalyst.
Informações legais
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Certificados de análise (COA)
Não está vendo a versão correta?
Se precisar de uma versão específica, você pode procurar um certificado específico pelo número do lote ou da remessa.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Artigos
The P-Phos ligand family was developed by Professor Chan of Hong Kong Polytechnic University and licensed to JM CCT in 2002. P-Phos is an atropisomeric biaryl bisphosphine with the unique feature of incorporating two methoxy-substituted pyridine rings in the backbone.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 675792-100MG | 4061833549650 |
| 675792-500MG | 4061833549667 |
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica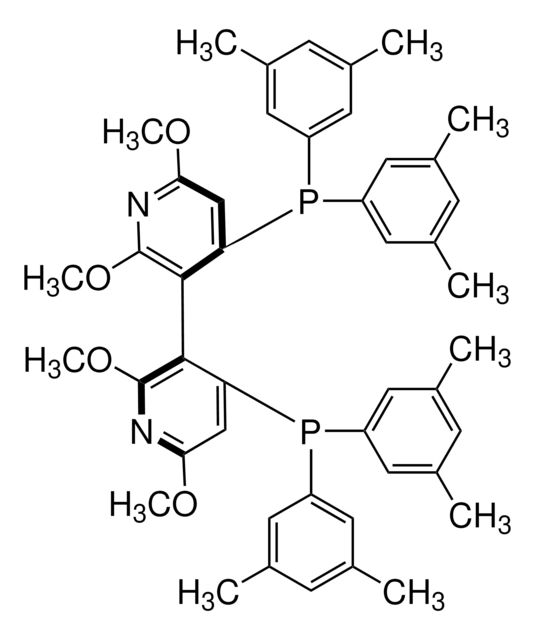
![(11bR,11′bR)-4,4′-(9,9-Dimethyl-9H-xanthene-4,5-diyl)bis-dinaphtho[2,1-d:1′, 2′-f][1,3,2]dioxaphosphepin](/deepweb/assets/sigmaaldrich/product/structures/198/331/bd88130d-f49e-4bc8-b82e-5e43b3bcea95/640/bd88130d-f49e-4bc8-b82e-5e43b3bcea95.png)

![Bis(tetrafluoroborato) de 1-clorometil-4-fluoro-1,4-diazoniabiciclo[2.2.2]octano >95% in F+ active](/deepweb/assets/sigmaaldrich/product/structures/206/487/53d52ee5-ef71-4e9a-9bc8-938b68b98d5d/640/53d52ee5-ef71-4e9a-9bc8-938b68b98d5d.png)