511188
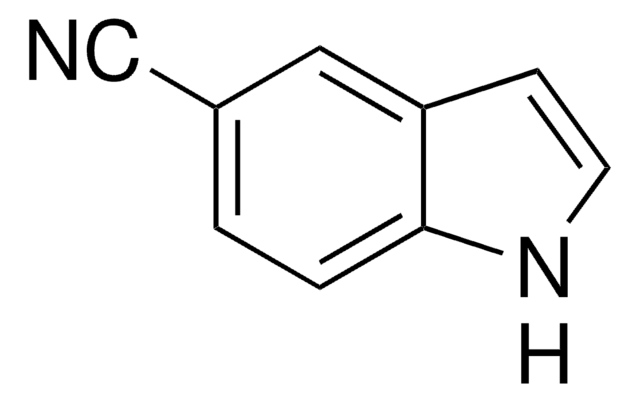
Methyl indole-5-carboxylate
99%
Sinônimo(s):
Indole-5-carboxylic acid, methyl ester
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C10H9NO2
Número CAS:
Peso molecular:
175.18
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
99%
pf
126-128 °C (lit.)
grupo funcional
ester
cadeia de caracteres SMILES
COC(=O)c1ccc2[nH]ccc2c1
InChI
1S/C10H9NO2/c1-13-10(12)8-2-3-9-7(6-8)4-5-11-9/h2-6,11H,1H3
chave InChI
DRYBMFJLYYEOBZ-UHFFFAOYSA-N
Descrição geral
Methyl indole-5-carboxylate (Methyl 1H-indole-5-carboxylate), a substituted 1H-indole, can be prepared by the esterification of indole-5-carboxylic acid. Its efficacy as a substrate for indigoid generation has been assessed.
Aplicação
Methyl indole-5-carboxylate may be used as a reactant in the following processes:
- biosynthesis of inhibitors of protein kinases
- metal-free Friedel-Crafts alkylation
- preparation of diphenylsulfonium ylides from Martin′s sulfurane
- cross dehydrogenative coupling reactions
- synthesis of indirubin derivatives
- preparation of aminoindolylacetates
Methyl indole-5-carboxylate may be used in the preparation of:
- methyl indoline-5-carboxylate
- 1H-indole-5-carbohydrazide
- dimethyl 1H-indole-3,5-dicarboxylate
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Wen-Jie Lu et al.
European journal of medicinal chemistry, 64, 498-511 (2013-05-21)
This report describes the synthesis, and in vitro and in vivo antimalarial evaluations of certain ester-modified neocryptolepine (5-methyl-5H-indolo[2,3-b]quinoline) derivatives. The modifications were carried out by introducing ester groups at the C2 and/or C9 position on the neocryptolepine core and the
Liu, Z.; et al.
Letters in Organic Chemistry, 7, 666-666 (2010)
Wang, T. C.; et al.
Chinese Chemical Letters = Zhongguo Hua Xue Kuai Bao, 21, 1407-1407 (2010)
Fei Yang et al.
Organic letters, 12(22), 5214-5217 (2010-10-23)
A novel cross dehydrogenative coupling (CDC) reaction of N,N-dimethylanilines with methyl ketones by cooperative copper and aminocatalysis has been developed, which leads to the formation of β-arylamino ketones in 42-73% yields. Moreover, the copper-catalyzed alkylation of free (NH) indoles with
A direct ylide transfer to carbonyl derivatives and heteroaromatic compounds.
Xueliang Huang et al.
Angewandte Chemie (International ed. in English), 49(47), 8979-8983 (2010-10-13)
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica