47714
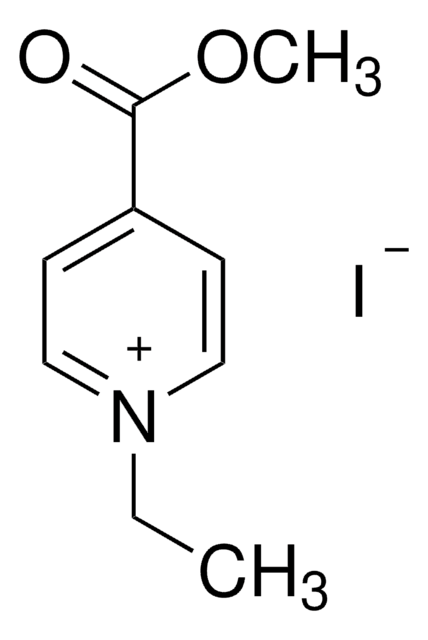
4-Formyl-1-methylpyridinium benzenesulfonate
≥95.0%
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C13H13NO4S
Número CAS:
Peso molecular:
279.31
Beilstein:
5695963
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
≥95.0%
forma
solid
Impurezas
≤2.0% water
pf
~95 °C
grupo funcional
aldehyde
sulfonic acid
cadeia de caracteres SMILES
[H]C(=O)c1cc[n+](C)cc1.[O-]S(=O)(=O)c2ccccc2
InChI
1S/C7H8NO.C6H6O3S/c1-8-4-2-7(6-9)3-5-8;7-10(8,9)6-4-2-1-3-5-6/h2-6H,1H3;1-5H,(H,7,8,9)/q+1;/p-1
chave InChI
HSVLGIFAXFDLMU-UHFFFAOYSA-M
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
4-Formyl-1-methylpyridinium benzenesulfonate is a pyridinium salt widely used for the conversion of primary amines to the carbonyl compounds like aldehydes and ketones. The reaction conditions are mild, suitable for compounds with sensitive functional groups thereby providing an efficient alternative for such transformations.
Aplicação
4-Formyl-1-methylpyridinium benzenesulfonate may be used as a reagent in the synthesis of the following:
- tetrazolic analogs of chalcones
- (+)-ferruginol
- Ecteinascidin 743
- Galipea alkaloids
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Jinchun Chen et al.
Journal of the American Chemical Society, 128(1), 87-89 (2006-01-05)
A convergent total synthesis of ecteinascidin 743 is realized from five building blocks of almost equal size. It takes 23 steps from l-3-hydroxy-4-methoxy-5-methyl phenylalanol (5) with an overall yield of 3%.
Ornella Mesenzani et al.
Bioorganic & medicinal chemistry letters, 21(2), 764-768 (2010-12-21)
In the chalcone scaffold, it is thought that the double bond is an important structural linker but it is likely not essential for the interaction with tubulin. Yet, it may be a potential site of metabolic degradation and interaction with
Zacharias Amara et al.
Natural product reports, 30(9), 1211-1225 (2013-07-31)
This review focuses on recent applications of the aza-Michael reaction in alkaloids total synthesis with a special emphasis on stereoselectivity. The report highlights achievements and challenges over the past five years and describes stereoselective intra- and inter-molecular conjugate addition of
Short syntheses of (+)-ferruginol from (+)-dehydroabietylamine.
Gonzalez MA and Perez-Guaita D.
Tetrahedron, 68(47), 9612-9615 (2012)
Mild and simple biomimetic conversion of amines to carbonyl compounds.
Buckley TF and Rapoport H
Journal of the American Chemical Society, 104(16), 4446-4450 (1982)
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica