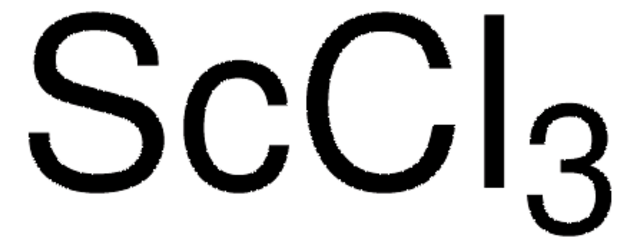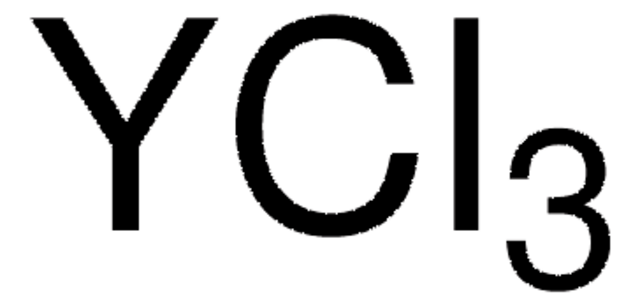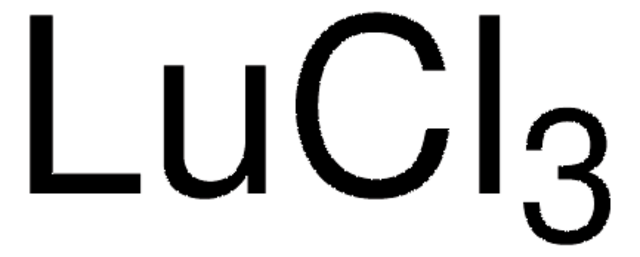451363
Yttrium(III) chloride
anhydrous, powder, 99.99% trace metals basis
Sinônimo(s):
Yttrium trichloride
About This Item
Produtos recomendados
grau
anhydrous
Nível de qualidade
Ensaio
99.99% trace metals basis
forma
powder
adequação da reação
reagent type: catalyst
core: yttrium
Impurezas
≤150.0 ppm Trace Rare Earth Analysis
pf
721 °C (lit.)
densidade
2.67 g/mL at 25 °C (lit.)
cadeia de caracteres SMILES
Cl[Y](Cl)Cl
InChI
1S/3ClH.Y/h3*1H;/q;;;+3/p-3
chave InChI
PCMOZDDGXKIOLL-UHFFFAOYSA-K
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Aplicação
- Efficient sky-blue perovskite light-emitting diodes via photoluminescence enhancement: This study demonstrates how adding yttrium (III) chloride to a perovskite mixture enhances photoluminescence quantum efficiency, significantly improving the performance of perovskite light-emitting diodes (Wang et al., 2019).
- Yttrium complexation and hydration in chloride-rich hydrothermal fluids: A combined study using molecular dynamics and X-ray absorption spectroscopy to understand yttrium′s complexation and hydration in chloride-rich hydrothermal environments (Guan et al., 2020).
Características e benefícios
acessório
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1 - Skin Sens. 1B
Código de classe de armazenamento
13 - Non Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Artigos
Advanced Inorganic Materials for Solid State Lighting
The rare earth elements impact nearly everyone in the world. All of the people living in advanced technological countries and almost all those living in third world countries utilize the rare earths in their everyday living—the car that one drives (gasoline is refined from oil using rare earth catalysts and catalytic converters reduce the polluting emissions from the automotive exhaust), watching the news on TV (the red and green colors in TV screens), the telephones and computers we use to communicate (the permanent magnets in speakers and disc drives), just to name a few examples.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica