About This Item
Fórmula empírica (Notação de Hill):
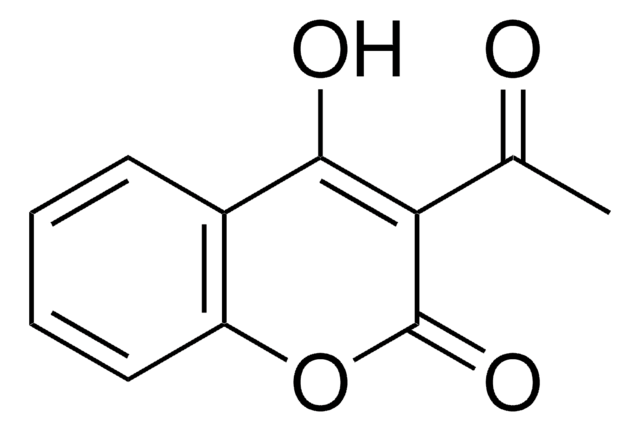
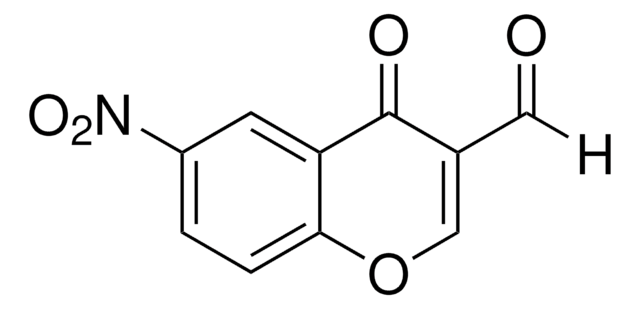
C9H5NO5
Número CAS:
Peso molecular:
207.14
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
98%
pf
172 °C (dec.) (lit.)
grupo funcional
ester
ketone
nitro
cadeia de caracteres SMILES
OC1=C(C(=O)Oc2ccccc12)[N+]([O-])=O
InChI
1S/C9H5NO5/c11-8-5-3-1-2-4-6(5)15-9(12)7(8)10(13)14/h1-4,11H
chave InChI
NZQAQAUWFHMVEM-UHFFFAOYSA-N
Descrição geral
4-Hydroxy-3-nitrocoumarin is a coumarin derivative and its cytotoxic action against cultured human tumor and normal cells has been investigated. It can be prepared by the nitration of 4-hydroxycoumarin in glacial acetic acid by using 72% HNO3.
Aplicação
4-Hydroxy-3-nitrocoumarin may be used as starting reagent for the synthesis of following compounds:
- 4-chloro-3-nitrocoumarin
- 2-unsubstituted 3-nitrochromone
- 4-amino-3-nitrocoumarins
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 3 Oral
Código de classe de armazenamento
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Biljana R Dekić et al.
Molecules (Basel, Switzerland), 15(4), 2246-2256 (2010-04-30)
Synthesis, spectral analysis and bioactivity of new coumarin derivatives are described in this paper. Eight new coumarin derivatives were synthesized in moderate to good yields by condensation of 4-chloro-3-nitrocoumarin and the corresponding heteroarylamine. The synthesized compounds were tested for their
Masami Kawase et al.
In vivo (Athens, Greece), 19(4), 705-711 (2005-07-08)
A preliminary exploration of coumarin derivatives as novel multidrug resistance (MDR) modulators was carried out to determine the basic features of the structure responsible for the MDR reversal activity. Among 44 coumarins, 14 compounds moderately induced the reversal of MDR
Vidoslav Dekić et al.
Magnetic resonance in chemistry : MRC, 48(11), 896-902 (2010-09-08)
Herein, we describe the synthesis and complete assignment of the (1)H and (13)C NMR chemical shifts of a series of antimicrobial 4-arylamino-3-nitrocoumarin derivatives based on a combination of (1)H and (13)C NMR, (1)H-(1)H-COSY, NOESY, HSQC and HMBC experiments. Conformational effects
Synthesis of heteroannulated 3-nitro-and 3-aminopyridines by cyclocondensation of electron-rich aminoheterocycles with 3-nitrochromone.
Iaroshenko VO, et al.
Tetrahedron, 68(11), 2532-2543 (2012)
Investigations of pyrans and related compounds.
Savel'ev VL, et al.
Chemistry of Heterocyclic Compounds, 9(7), 816-820 (1973)
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica