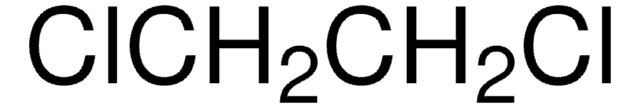About This Item
Fórmula empírica (Notação de Hill):
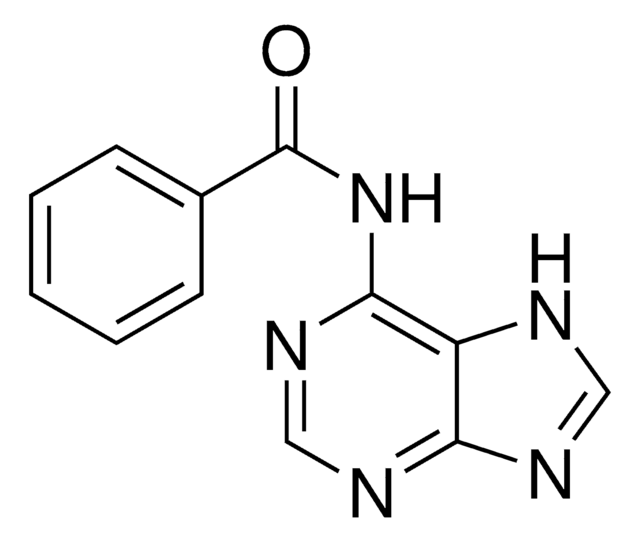
C11H9N3O2
Número CAS:
Peso molecular:
215.21
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Ensaio
98%
pf
>300 °C (dec.) (lit.)
grupo funcional
amide
phenyl
cadeia de caracteres SMILES
O=C1NC=CC(NC(=O)c2ccccc2)=N1
InChI
1S/C11H9N3O2/c15-10(8-4-2-1-3-5-8)13-9-6-7-12-11(16)14-9/h1-7H,(H2,12,13,14,15,16)
chave InChI
XBDUZBHKKUFFRH-UHFFFAOYSA-N
Descrição geral
N4-Benzoylcytosine is an amide and its anti-microbial activity against pathogenic microorganisms has been studied using the Disk Diffusion and the Pour Plate method. It can be synthesized via the condensation of benzoyl chloride with cytosine.
Aplicação
N4-Benzoylcytosine may be employed for the following syntheses:
- 3′-C-ethynyl and 3′-C-(1,4-disubstituted-1,2,3-triazolo) double-headed pyranonucleosides
- 2′-C-methyl-4′-thiocytidine, via the Pummerer reaction
- 2′-fluorinated L-nucleoside analogs
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Christos Kiritsis et al.
Medicinal chemistry (Shariqah (United Arab Emirates)), 8(3), 320-329 (2012-04-26)
A novel series of 3'-C-ethynyl and 3'-C-(1,4-disubstituted-1,2,3-triazolo) double-headed pyranonucleosides has been designed and synthesized. Reaction of 3-keto glucoside 1 with ethynyl magnesium bromide gave the desired precursor 3-C-ethynyl-1,2:5,6-di-O-isopropylidene-α-D-allofuranose (2). Hydrolysis followed by acetylation led to the 1,2,4,6-tetra-O-acetyl-3-C-ethynyl-β-D-allopyranose (3). Compound 3
Daisuke Kaga et al.
Nucleosides, nucleotides & nucleic acids, 24(10-12), 1789-1800 (2006-01-28)
The synthesis of 2'-C-methyl-4'-thiocytidine (16) is described. Since the 2'-keto-4'-thiocytidine derivative 2beta unexpectedly isomerized to 2alpha and the methylation of 2beta proceeded predominantly from the less hindered alpha-face to give 7, the desired product 16 was synthesized via the Pummerer
Antimicrobial activity of amide, N4-benzoylcytosine.
Jagessar RC and Gomathinayagam S.
Journal of Pharmacy and Clinical Sciences, 1, 12-19 (2011)
K Lee et al.
Journal of medicinal chemistry, 42(7), 1320-1328 (1999-04-10)
The synthesis of L-nucleoside analogues containing 2'-vinylic fluoride was accomplished by direct condensation method, and their anti-HIV and anti-HBV activities were evaluated in vitro. The key intermediate 8, the sugar moiety of our target compounds, was prepared from 1,2-O-isopropylidene-L-glyceraldehyde via
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica