About This Item
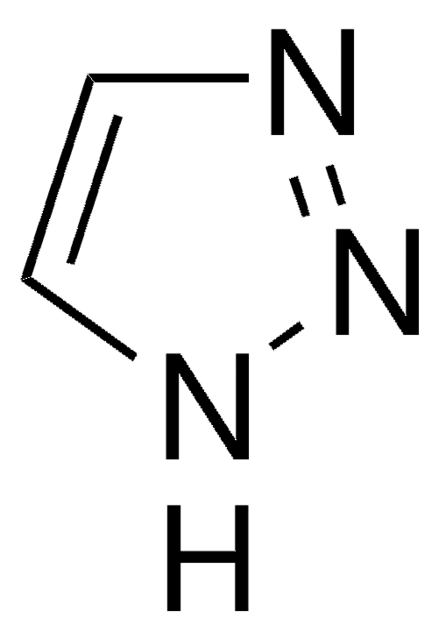
Fórmula empírica (Notação de Hill):
C4H3F3N2
Número CAS:
Peso molecular:
136.08
Beilstein:
1564179
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Ensaio
99%
pb
70 °C/2 mmHg (lit.)
pf
45-47 °C (lit.)
cadeia de caracteres SMILES
FC(F)(F)c1cc[nH]n1
InChI
1S/C4H3F3N2/c5-4(6,7)3-1-2-8-9-3/h1-2H,(H,8,9)
chave InChI
PYXNITNKYBLBMW-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
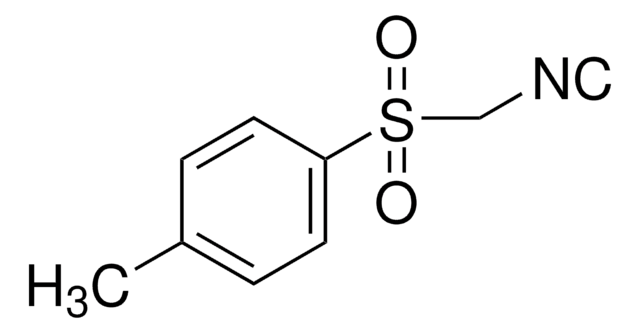
3-(Trifluoromethyl)pyrazoles is a heterocyclic building block. It undergoes alkylation with alkyl iodides in DMF to afford the N-alkyl pyrazoles. It participates in the synthesis of disubstituted pyrimidines.
Aplicação
3-(Trifluoromethyl)pyrazole may be used in copper-catalyzed pyrazole N-arylation. It may be used in the synthesis of sodium hydridotris(1H-3-trifluoromethylpyrazol-1-yl)borate by heating with sodium borohydride.
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
[A simple method for the direct preparation of O2,3'-cyclo-2'-desoxynucleosides].
G Kowollik et al.
Tetrahedron letters, 44(44), 3863-3865 (1969-09-01)
The effect of the 3-trifluoromethyl substituent in polypyrazolylborato complexes on the iron(II) spin state; X-ray diffraction and absorption and Mossbauer studies.
Cecchi P, et al.
Inorgorganica Chimica Acta, 318(1), 67-76 (2001)
Mild Conditions for Copper-Catalysed N-Arylation of Pyrazoles.
Cristau HJ, et al.
European Journal of Organic Chemistry, 4, 695-709 (2004)
Wei Zhang
Organic letters, 5(7), 1011-1013 (2003-03-28)
[reaction: see text] The fluorous synthesis of disubstituted pyrimidines is carried out by attaching 2,4-dichloro-6-methylpyrimidine with 1H,1H,2H,2H-perfluorodecanethiol. The tagged substrate is substituted with 3-(trifluoromethyl)pyrazole followed by thioether oxidation and tag displacement with amines or thiols. The fluorous chain serves as
George P Lahm et al.
Bioorganic & medicinal chemistry letters, 15(22), 4898-4906 (2005-09-17)
A novel class of anthranilic diamides has been discovered with exceptional insecticidal activity on a range of Lepidoptera. These compounds have been found to exhibit their action by release of intracellular Ca2+ stores mediated by the ryanodine receptor. The discovery
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica


![1H-1,2,3-Triazolo[4,5-b]pyridine 98%](/deepweb/assets/sigmaaldrich/product/structures/344/744/1e7fa2cf-1258-48e0-909f-92509981f43d/640/1e7fa2cf-1258-48e0-909f-92509981f43d.png)