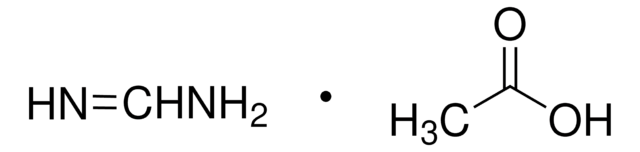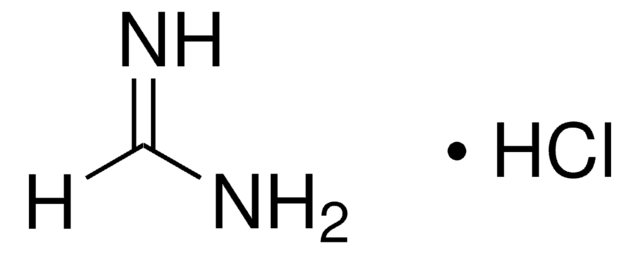396788
Ethyl formimidate hydrochloride
Sinônimo(s):
Ethyl methanimidate hydrochloride
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula linear:
HC(=NH)OC2H5·HCl
Número CAS:
Peso molecular:
109.55
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Formulário
solid
Nível de qualidade
pf
75 °C (dec.) (lit.)
grupo funcional
ether
temperatura de armazenamento
2-8°C
cadeia de caracteres SMILES
Cl.CCOC=N
InChI
1S/C3H7NO.ClH/c1-2-5-3-4;/h3-4H,2H2,1H3;1H
chave InChI
JPUTTYRVDANTBN-UHFFFAOYSA-N
Descrição geral
Ethyl formimidate hydrochloride is a nitrogen containing organic building block. On heating it undergoes degradation to afford formamidine hydrochloride, ethyl formate and ethyl chloride.
Aplicação
Ethyl formimidate hydrochloride may be used in the preparation of the following:
- 5-aminoimidazole-4-carboxylic acid α- and β-ribotides
- s-triazines
- 2,3:5,6-di-O-isopropylidene-α- and -β-5-amino-4-ethoxycarbonyl or -carbamoyl-imidazole D-mannofuranosides
- 5-amino-1-(2-pyridyl)imidazole
Reactant involved in the synthesis of biologically active molecules including:
Reactant involved in:
- Bredinin via amination of an acyclic precursor
- Amidine conjugates of the ornithine moiety of an antifungal
Reactant involved in:
- Intermolecular cyclization
- Mo-catalyzed asymmetric ring-closing metathesis for synthesis of cyclid amides and amines
- Synthesis of peptidic 1-cyanopyrrolidines
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Purines, pyrimidines, and imidazoles part 54. Interconversion of some intermediates in the de novo biosynthesis of purine nucleotides.
Cusack NJ, et al.
Journal of the Chemical Society. Perkin Transactions 1, 2316-2321 (1980)
Purines, pyrimidines, and imidazoles. Part XLI. Glycofuranosylamines derived from D-xylose, D-glucose, D-mannose, and L-rhamnose and their use in the synthesis of pyrimidine and imidazole nucleosides.
Cusack NJ, et al.
Journal of the Chemical Society. Perkin Transactions 1, 73-81 (1974)
Purines, pyrimidines, and imidazoles. XL. A new synthesis of a D-ribofuranosylamine derivative and its use in the synthesis of pyrimidine and imidazole nucleosides.
N J Cusack et al.
Journal of the Chemical Society. Perkin transactions 1, 16, 1720-1731 (1973-01-01)
Synthesis of the S-Triazine System. Iii. 1 Trimerization of Imidates.
Schaefer FC and Peters GA.
The Journal of Organic Chemistry, 26(8), 2771-2784 (1961)
Rhodd EH and von Richter V.
Chemistry of Organic Compounds: Pt. B. Aliphatic compounds, 551-551 (1951)
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica