392251
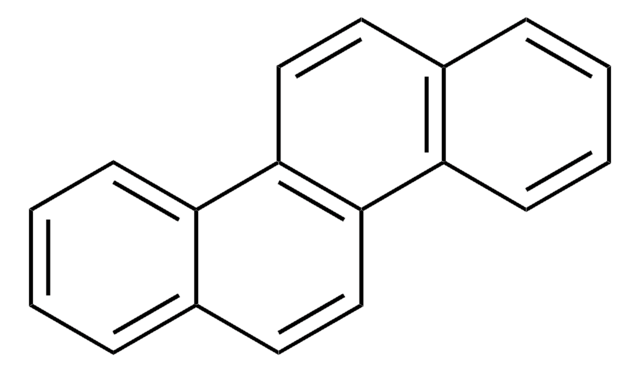
Benzo[k]fluoranthene
for fluorescence, ≥99%
Sinônimo(s):
11,12-Benzofluoranthene, 2,3,1′,8′-Binaphthylene, 8,9-Benzfluoranthene
About This Item
Produtos recomendados
grau
for fluorescence
Ensaio
≥99%
pf
215-217 °C (lit.)
solubilidade
95% ethanol: <1 mg/mL at 20 °C
DMSO: <1 mg/mL at 20 °C
H2O: <1 mg/mL at 20 °C
acetone: 1-10 mg/mL at 20 °C
methanol: <1 mg/mL at 20 °C
toluene: 5-10 mg/mL at 20 °C
cadeia de caracteres SMILES
c1ccc2cc-3c(cc2c1)-c4cccc5cccc-3c45
InChI
1S/C20H12/c1-2-6-15-12-19-17-10-4-8-13-7-3-9-16(20(13)17)18(19)11-14(15)5-1/h1-12H
chave InChI
HAXBIWFMXWRORI-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Aplicação
- Enhanced degradation of carcinogenic PAHs benzo (a) pyrene and benzo (k) fluoranthene by a microbial consortia: Bioremediation of high molecular weight PAHs with a combination of microorganisms (S Guntupalli, V Thunuguntla, 2016).
- Biotransformation of the high‐molecular weight polycyclic aromatic hydrocarbon (PAH) benzofluoranthene by Sphingobium sp. strain KK22 and identification of metabolites: Biotransformation and identification of products from benzo[k]fluoranthene (AH Maeda, S Nishi, Y Hatada, Y Ozeki, 2014).
- Investigation of the electrochemical properties of benzofluorenthene using a glassy carbon electrode and development of a square-wave voltammetric method for detection: Electrochemical behavior of benzo[k]fluorenthene and development of detection method (A Altun, Y Yardim, A Levent, 2023).
Embalagem
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 1B
Código de classe de armazenamento
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe de risco de água (WGK)
WGK 3
Equipamento de proteção individual
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica![Benzo[b]fluoranthene 98%](/deepweb/assets/sigmaaldrich/product/structures/175/744/6fa5fca2-b6ec-47b6-ab7a-fe895843f226/640/6fa5fca2-b6ec-47b6-ab7a-fe895843f226.png)
![Benzo[ghi]perylene 98%](/deepweb/assets/sigmaaldrich/product/structures/154/740/c50ff1be-dfb4-4159-a98c-9cecf9206ad3/640/c50ff1be-dfb4-4159-a98c-9cecf9206ad3.png)
![Indeno[1,2,3-cd]pyrene analytical standard](/deepweb/assets/sigmaaldrich/product/structures/231/153/b0b230c2-efa0-4f43-a261-66b931ead3d2/640/b0b230c2-efa0-4f43-a261-66b931ead3d2.png)
![Dibenz[a,h]anthracene certified reference material, TraceCERT®, Manufactured by: Sigma-Aldrich Production GmbH, Switzerland](/deepweb/assets/sigmaaldrich/product/structures/358/871/0a80ecfc-d123-44ca-90a6-22248b43aba9/640/0a80ecfc-d123-44ca-90a6-22248b43aba9.png)
![Benzo[a]pyrene ≥96% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/253/820/be96d879-1811-46c0-8f11-612019691c2d/640/be96d879-1811-46c0-8f11-612019691c2d.png)

![Benzo[k]fluoranthene certified reference material, TraceCERT®, Manufactured by: Sigma-Aldrich Production GmbH, Switzerland](/deepweb/assets/sigmaaldrich/product/structures/277/320/3e615f9f-3887-40f6-b176-bc1eb9b4832c/640/3e615f9f-3887-40f6-b176-bc1eb9b4832c.png)