366978
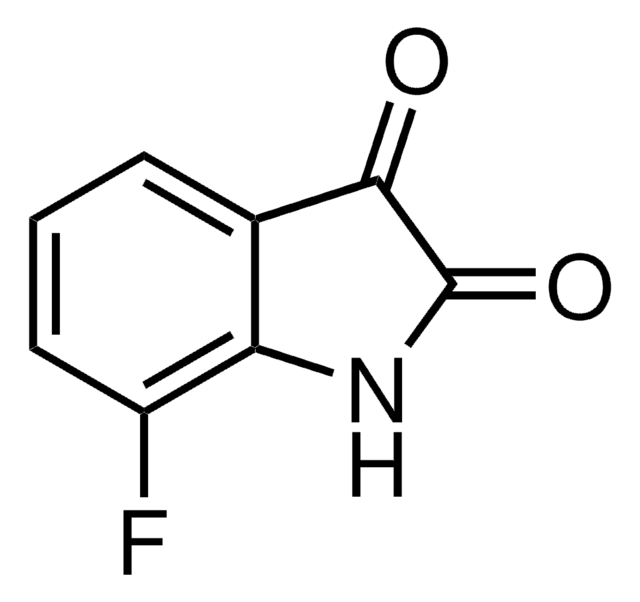
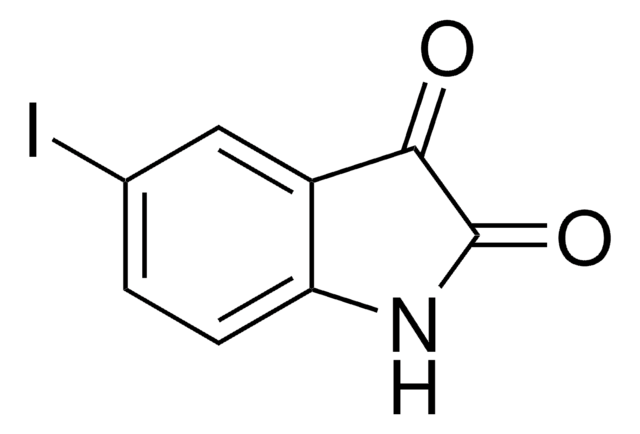
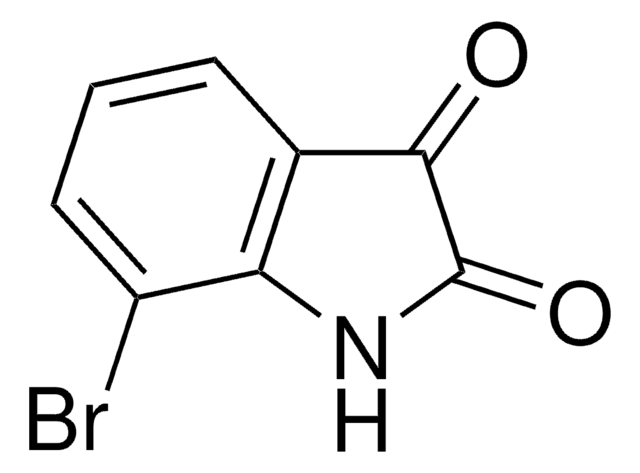
5-Fluoroisatin
98%
Sinônimo(s):
5-Fluoro-2,3-indoledione, NSC 39161
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C8H4FNO2
Número CAS:
Peso molecular:
165.12
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Ensaio
98%
pf
224-227 °C (lit.)
grupo funcional
fluoro
ketone
cadeia de caracteres SMILES
Fc1ccc2NC(=O)C(=O)c2c1
InChI
1S/C8H4FNO2/c9-4-1-2-6-5(3-4)7(11)8(12)10-6/h1-3H,(H,10,11,12)
chave InChI
GKODDAXOSGGARJ-UHFFFAOYSA-N
Descrição geral
5-Fluoroisatin has been reported as the precursor of the Sunitinib (Sutent) drug. 5-Fluoroisatin has been approved by the Food and Drugs Administration (FDA) in 2006 for the treatment of renal cell carcinoma (RCC) and gastrointestinal stromal tumor (GIST).
Aplicação
5-Fluoroisatin may be used:
- as reaction-based probe for live-cell detection of peroxynitrite by 19F magnetic resonance spectroscopy
- in non-invasive detection of peroxynitrite (ONOO(-)) formation in living lung epithelial cells stimulated with interferon-γ (IFN-γ)
- in the synthesis of bis-Schiff bases, via condensation with aromatic primary bis-amines in water suspension medium without using any organic solvent or acid catalyst
- in the synthesis of 3-acetonyl-5-fluoro-3-hydroxyoxindole
Reactant for preparation of:
- Spiro[indole-thiazolidinones] as biologically relevan synthesis scaffolds
- Potential antimycobacterial agents
- Inhibitors of c-Met kinase
- Inhibitors of TAK1 kinase
- Herpes simplex virus inhibitors
- IKKβ inhibitors
- Inhibitors of vitiligo disease
- Potential drug candidates with anti-HIV activity and anti-tubercular activity
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
A Novel Preparation of a-Substituted Tryptamines from Isatins.
Franklin CS and White AC.
Journal of the Chemical Society, 2, 1335-1337 (1963)
A A Jarrahpour et al.
Molecules (Basel, Switzerland), 11(1), 59-63 (2007-10-27)
Condensation of aromatic primary bis-amines with isatin (1H-indole-2,3-dione) and 5-flouroisatin occurred cleanly and efficiently in a water suspension medium without using any organic solvent or acid catalyst. The corresponding bis-Schiff bases were obtained in good yields and were easily isolated
Counter-Current chromatography separation of isatin derivatives using the sandmeyer methodology.
Almeida MR, et al.
Journal of the Brazilian Chemical Society, 21(4), 764-769 (2010)
Kevin J Bruemmer et al.
Chemical communications (Cambridge, England), 50(82), 12311-12314 (2014-09-03)
We report a newly discovered oxidative decarbonylation reaction of isatins that is selectively mediated by peroxynitrite (ONOO(-)) to provide anthranilic acid derivatives. We have harnessed this rapid and selective transformation to develop two reaction-based probes, 5-fluoroisatin and 6-fluoroisatin, for the
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica