271438
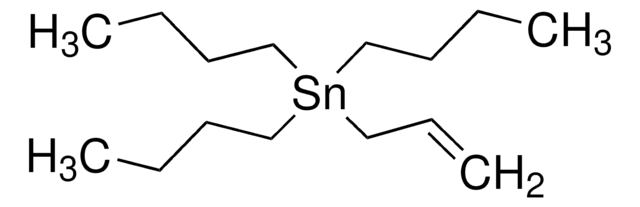
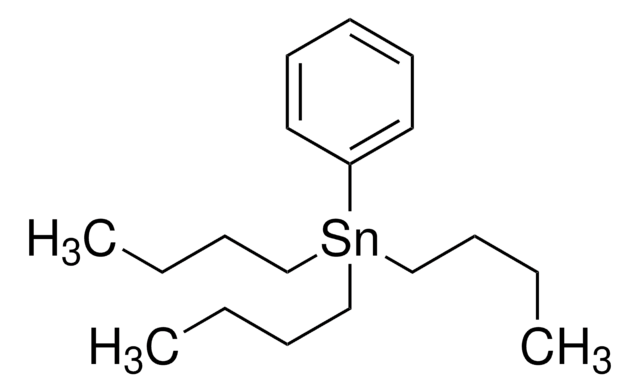
Tributyl(vinyl)tin
97%
Sinônimo(s):
Tributyl(vinyl)stannane, Tributylstannylethylene, Vinyltributylstannane
About This Item
Produtos recomendados
Ensaio
97%
índice de refração
n20/D 1.478 (lit.)
pb
104-106 °C/3.5 mmHg (lit.)
densidade
1.085 g/mL at 25 °C (lit.)
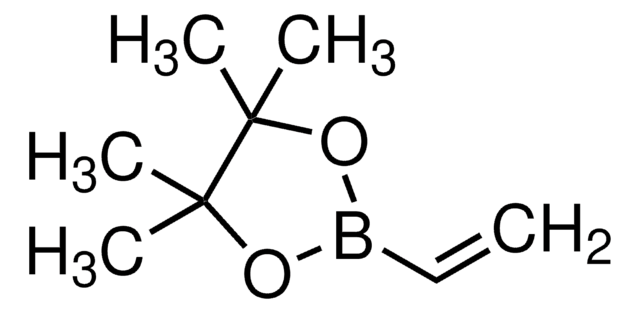
cadeia de caracteres SMILES
CCCC[Sn](CCCC)(CCCC)C=C
InChI
1S/3C4H9.C2H3.Sn/c3*1-3-4-2;1-2;/h3*1,3-4H2,2H3;1H,2H2;
chave InChI
QIWRFOJWQSSRJZ-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Descrição geral
Aplicação
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 3 Oral - Acute Tox. 4 Dermal - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Flam. Liq. 3 - Repr. 1B - Skin Irrit. 2 - STOT RE 1
Código de classe de armazenamento
3 - Flammable liquids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
109.4 °F - closed cup
Ponto de fulgor (°C)
43 °C - closed cup
Equipamento de proteção individual
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Artigos
We carry a large variety of electrophiles and nucleophiles that are widely used in C–C bond-forming reactions. This group of products contains many organometallic reagents as well as commonly-used alkylating and acylating reagents.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)

![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II), complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)