About This Item
Fórmula empírica (Notação de Hill):
C14H9Cl2NO
Número CAS:
Peso molecular:
278.13
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
97%
forma
solid
pf
163-165 °C (lit.)
grupo funcional
chloro
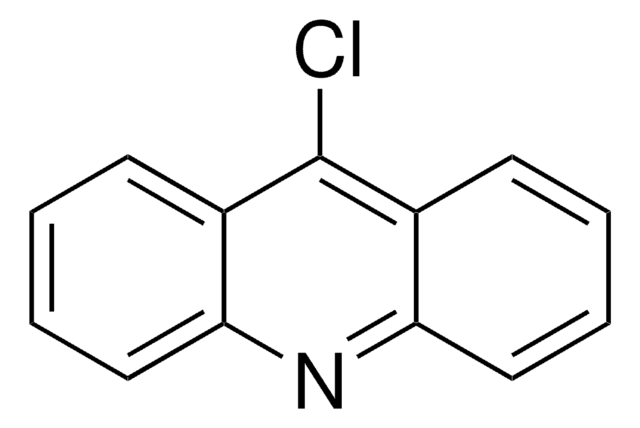
cadeia de caracteres SMILES
COc1ccc2nc3cc(Cl)ccc3c(Cl)c2c1
InChI
1S/C14H9Cl2NO/c1-18-9-3-5-12-11(7-9)14(16)10-4-2-8(15)6-13(10)17-12/h2-7H,1H3
chave InChI
RYRNQWYNHLLOGX-UHFFFAOYSA-N
Descrição geral
6,9-Dichloro-2-methoxyacridine on reaction with quinolizidinylalkylamines yields 4-aminoquinoline and 9-aminoacridine derivatives.
Aplicação
6,9-Dichloro-2-methoxyacridine was used in the synthesis of 9-amino-6-chloro-2-methoxyacridine, N′-(6-Chloro-2-methoxy-acridin-9-yl)-heptylamine and N,N′-bis-(6-chloro-2-methoxy-acridin-9-yl)-hexane-1,6-diamine.
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Origin of the complex fluorescence emission of 9-amino-6-chloro-2-methoxyacridine. 1. Experiment.
Fan P, et al.
The Journal of Physical Chemistry, 93(18), 6615-6622 (1989)
C Boido Canu et al.
Bollettino chimico farmaceutico, 128(6), 212-215 (1989-06-01)
By reacting three quinolizidinylalkylamines with 4,7-dichloroquinoline and 6,9-dichloro-2-methoxyacridine six derivatives of 4-aminoquinoline and 9-aminoacridine were obtained. These compounds, which are of interest as potential antibacterial, antiprotozoarian, anti-helminthic and antitumoral agents, so far have been tested against lymphocytic leukemia P 388
Lucie Guetzoyan et al.
Bioorganic & medicinal chemistry, 17(23), 8032-8039 (2009-11-03)
A series of acridine derivatives were synthesised and their in vitro antimalarial activity was evaluated against one chloroquine-susceptible strain (3D7) and three chloroquine-resistant strains (W2, Bre1 and FCR3) of Plasmodium falciparum. Structure-activity relationship showed that two positives charges as well
Ana Gomes et al.
ChemMedChem, 9(2), 305-310 (2014-01-30)
Plasmodium falciparum, the causative agent of the most lethal form of malaria, is becoming increasingly resistant to most available drugs. A convenient approach to combat parasite resistance is the development of analogues of classical antimalarial agents, appropriately modified in order
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica