About This Item
Fórmula empírica (Notação de Hill):
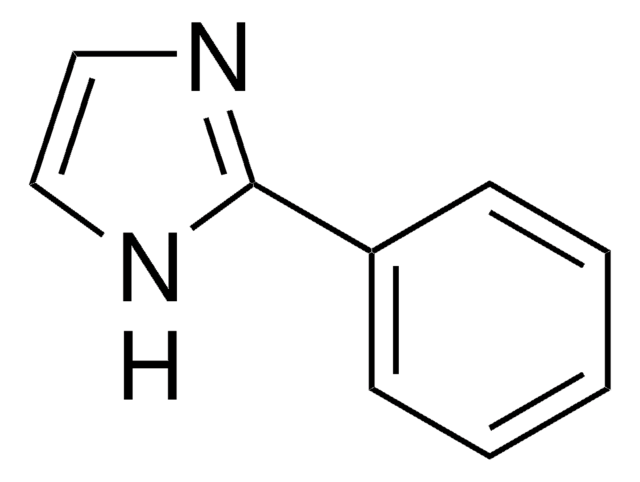
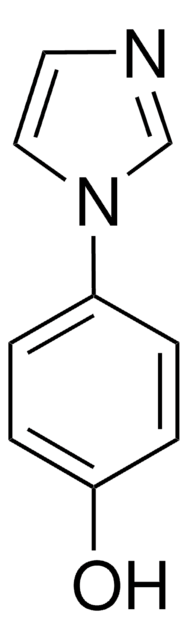
C9H8N2
Número CAS:
Peso molecular:
144.17
Beilstein:
2969
Número CE:
Número MDL:
Código UNSPSC:
12352005
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Ensaio
97%
pf
128-131 °C (lit.)
solubilidade
acetone: soluble 25 mg/mL, clear, colorless to yellow (typical)
grupo funcional
phenyl
cadeia de caracteres SMILES
c1ccc(cc1)-c2c[nH]cn2
InChI
1S/C9H8N2/c1-2-4-8(5-3-1)9-6-10-7-11-9/h1-7H,(H,10,11)
chave InChI
XHLKOHSAWQPOFO-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Aplicação
4-Phenylimidazole was used to investigate the protein-ligand interactions in cytochrome P450 from the thermoacidophile Picrophilus torridus. It was used as heme ligand during the crystallization of recombinant human indoleamine 2,3-dioxygenase. It was used in the synthesis of complexes of copper and cobalt.
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
M Spatzenegger et al.
Molecular pharmacology, 59(3), 475-484 (2001-02-17)
The molecular basis for reversible inhibition of rabbit CYP2B4 and CYP2B5 and rat CYP2B1 by phenylimidazoles was assessed with active-site mutants and new three-dimensional models based on the crystal structure of CYP2C5. 4-Phenylimidazole was 17- to 32-fold more potent toward
Danni L Harris et al.
Proteins, 55(4), 895-914 (2004-05-18)
The molecular origins of temperature-dependent ligand-binding affinities and ligand-induced heme spin state conversion have been investigated using free energy analysis and DFT calculations for substrates and inhibitors of cytochrome P450 2B4 (CYP2B4), employing models of CYP2B4 based on CYP2C5(3LVdH)/CYP2C9 crystal
Y K Li et al.
Biochimica et biophysica acta, 999(3), 227-232 (1989-12-21)
Over 25 nitrogen-containing heterocycles were tested as inhibitors of sweet almond beta-glucosidase (EC 3.2.1.21). Among the most potent of these are some imidazole derivatives. The pH dependence indicates that the unprotonated inhibitor binds most tightly to the catalytically active species
Spectral and metabolic properties of liver microsomes from imidazole-pretreated rabbits.
K K Hajek et al.
Biochemical and biophysical research communications, 108(2), 664-672 (1982-09-30)
Y K Li et al.
Journal of biochemistry, 123(3), 416-422 (1998-05-30)
Series of 4-arylimidazoles, omega-N-acylhistamines and 4-(omega-phenylalkyl)imidazoles were synthesized in order to probe the active site topology of sweet almond beta-glucosidase. These imidazole derivatives were shown to be very powerful competitive inhibitors. Among the 20 tested compounds, omega-N-benzoylhistamine and 4-(3'-phenylpropyl)imidazole are
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica