126152
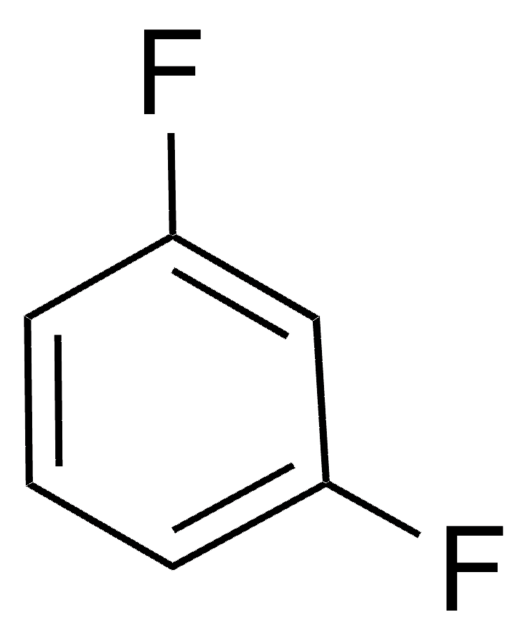
1,2-Difluorobenzene
98%
Sinônimo(s):
o-Difluorobenzene
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C6H4F2
Número CAS:
Peso molecular:
114.09
Beilstein:
1905113
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
98%
Formulário
liquid
índice de refração
n20/D 1.443 (lit.)
p.e.
92 °C (lit.)
pf
−34 °C (lit.)
densidade
1.158 g/mL at 25 °C (lit.)
grupo funcional
fluoro
cadeia de caracteres SMILES
Fc1ccccc1F
InChI
1S/C6H4F2/c7-5-3-1-2-4-6(5)8/h1-4H
chave InChI
GOYDNIKZWGIXJT-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Descrição geral
1,2-Difluorobenzene undergoes defluorination under very mild conditions by H2 in the presence of NaOAc over rhodium pyridylphosphine and bipyridyl complexes tethered on a silica-supported palladium catalyst.
Aplicação
1,2-Difluorobenzene(1,2-DFB) has been used to study the mechanism of dissociation of o-, m- and p-difluorobenzene ions by threshold photoelectron photoion coincidence spectroscopy. It has been used to study the room temperature adsorption of 1,2-DFB, 1,2-dichlorobenzene and 1,2-dibromobenzene on Si(100)2x1 by X-ray photoelectron spectroscopy and temperature programmed desorption. It was used as solvent in electrochemical studies on transition metal complexes.
Solvent useful for electrochemical studies on transition metal complexes.
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Flam. Liq. 2
Código de classe de armazenamento
3 - Flammable liquids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
33.8 °F - closed cup
Ponto de fulgor (°C)
1 °C - closed cup
Equipamento de proteção individual
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Hydrodefluorination of fluorobenzene and 1, 2-difluorobenzene under mild conditions over rhodium pyridylphosphine and bipyridyl complexes tethered on a silica-supported palladium catalyst.
Yang H, et al.
Organometallics, 18(12), 2285-2287 (1999)
Competition between associative and dissociative adsorption of 1, 2-dihalogenated benzenes on Si (100) 2? 1: Formation of dihalocyclohexadiene, halophenyl and phenylene adstructures.
Zhou XJ and Leung KT.
Surface Science, 600(16), 3285-3296 (2006)
Anne-Marie Boulanger et al.
Journal of the American Society for Mass Spectrometry, 20(1), 20-24 (2008-10-18)
Threshold photoelectron photoion coincidence (TPEPICO) experiments have shown that o-, m-, and p-difluorobenzene ions dissociate via a common, ring-opened intermediate and not via ionized p-difluorobenzene. Rice-Ramsperger-Kassel-Marcus (RRKM) modeling of the experimental breakdown curves yields activation energies for the initial isomerization
Inorganic Chemistry, 28, 3923-3923 (1989)
Adrian Romero-Flores et al.
Chemosphere, 186, 151-159 (2017-08-05)
Electronic noses have been widely used in the food industry to monitor process performance and quality control, but use in wastewater and biosolids treatment has not been fully explored. Therefore, we examined the feasibility of an electronic nose to discriminate
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 126152-10G | 4061838723871 |
| 126152-50G | 4061838723888 |
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica