122459
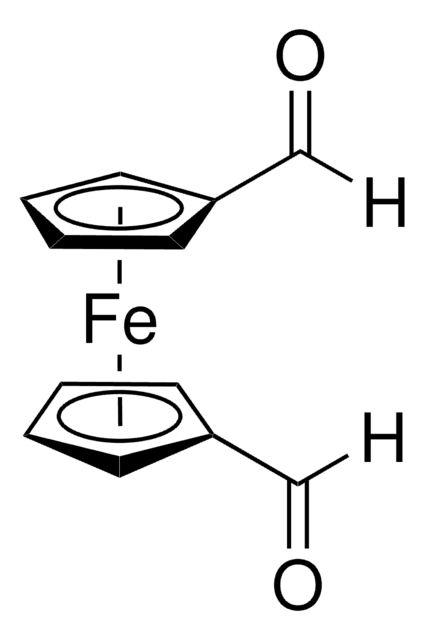
Ferrocenecarboxaldehyde
98%
Sinônimo(s):
Cyclopentadienyl(formylcyclopentadienyl)iron
About This Item
Produtos recomendados
Nível de qualidade
Ensaio
98%
Formulário
solid
adequação da reação
core: iron
reagent type: catalyst
pf
118-120 °C (lit.)
grupo funcional
aldehyde
temperatura de armazenamento
2-8°C
cadeia de caracteres SMILES
[Fe].[CH]1[CH][CH][CH][CH]1.[H]C(=O)[C]2[CH][CH][CH][CH]2
InChI
1S/C6H5O.C5H5.Fe/c7-5-6-3-1-2-4-6;1-2-4-5-3-1;/h1-5H;1-5H;
chave InChI
UQTCQJVPLIVCAX-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Aplicação
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 122459-5G | 4061838721259 |
| 122459-1G | |
| 122459-25G | 4061838721242 |
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica