E1383
Etoposide
95.0-105.0% (anhydrous basis), powder, topoisomerase II inhibitor
Synonyme(s) :
4′-Demethylepipodophyllotoxin 9-(4,6-O-ethylidene-β-D-glucopyranoside), VP-16-213
About This Item
Produits recommandés
product name
Etoposide, synthetic, 95.0-105.0%, powder
Source biologique
synthetic
Niveau de qualité
Pureté
95.0-105.0%
Forme
powder
Couleur
white to off-white
pKa
9.8
Pf
236-251 °C (lit.)
Spectre d'activité de l'antibiotique
neoplastics
Mode d’action
DNA synthesis | interferes
enzyme | inhibits
Auteur
Teva
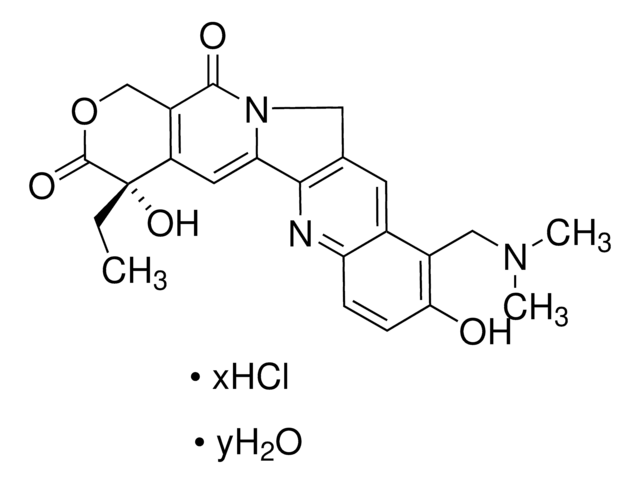
Chaîne SMILES
COc1cc(cc(OC)c1O)[C@H]2[C@@H]3[C@H](COC3=O)[C@H](O[C@@H]4O[C@@H]5CO[C@@H](C)O[C@H]5[C@H](O)[C@H]4O)c6cc7OCOc7cc26
InChI
1S/C29H32O13/c1-11-36-9-20-27(40-11)24(31)25(32)29(41-20)42-26-14-7-17-16(38-10-39-17)6-13(14)21(22-15(26)8-37-28(22)33)12-4-18(34-2)23(30)19(5-12)35-3/h4-7,11,15,20-22,24-27,29-32H,8-10H2,1-3H3/t11-,15+,20-,21-,22+,24-,25-,26-,27-,29+/m1/s1
Clé InChI
VJJPUSNTGOMMGY-MRVIYFEKSA-N
Informations sur le gène
human ... ABCB1(5243) , CYP3A4(1576) , TOP2A(7153) , TOP2B(7155)
mouse ... Abcb1a(18671) , Abcb1b(18669)
rat ... Top2a(360243)
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
- to prepare drug stock solution in dimethyl sulfoxide (DMSO) and also to profile and compare the sensitivity of DT40 mutant cells
- to incubate cells for cell viability assay
- to treat neuro-2A cells to induce programmed cell death
Actions biochimiques/physiologiques
Caractéristiques et avantages
Conditionnement
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Oral - Carc. 1B - Repr. 2
Code de la classe de stockage
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe de danger pour l'eau (WGK)
WGK 3
Équipement de protection individuelle
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
We presents an article on ABC Transporters and Cancer Drug Resistance
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique