C1613
Carbenicillin
Ready Made Solution, 100 mg/mL in ethanol/water, 0.2 μm filtered
Synonyme(s) :
α-Carboxybenzylpenicillin solution
About This Item
Produits recommandés
Niveau de qualité
Stérilité
0.2 μm filtered
Forme
liquid
Concentration
100 mg/mL in ethanol/water
Solubilité
H2O: 100 mg/mL
ethanol: 100 mg/mL
Adéquation
suitable for testing specifications (Bacteriostatic (cell type) E. Coli DH5a, result: pass)
Spectre d'activité de l'antibiotique
Gram-negative bacteria
Gram-positive bacteria
Mode d’action
cell wall synthesis | interferes
Conditions d'expédition
wet ice
Température de stockage
−20°C
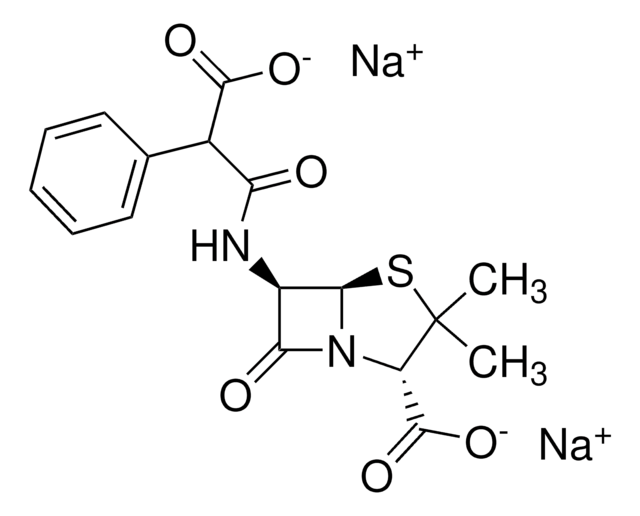
Chaîne SMILES
CC1(C)S[C@@H]2[C@H](NC(=O)C(C(O)=O)c3ccccc3)C(=O)N2[C@H]1C(O)=O
InChI
1S/C17H18N2O6S/c1-17(2)11(16(24)25)19-13(21)10(14(19)26-17)18-12(20)9(15(22)23)8-6-4-3-5-7-8/h3-7,9-11,14H,1-2H3,(H,18,20)(H,22,23)(H,24,25)/t9?,10-,11+,14-/m1/s1
Clé InChI
FPPNZSSZRUTDAP-UWFZAAFLSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
Actions biochimiques/physiologiques
Autres remarques
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Flam. Liq. 3 - Resp. Sens. 1 - Skin Sens. 1
Code de la classe de stockage
3 - Flammable liquids
Classe de danger pour l'eau (WGK)
WGK 2
Point d'éclair (°F)
104.0 °F - closed cup
Point d'éclair (°C)
40 °C - closed cup
Équipement de protection individuelle
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique