A4487
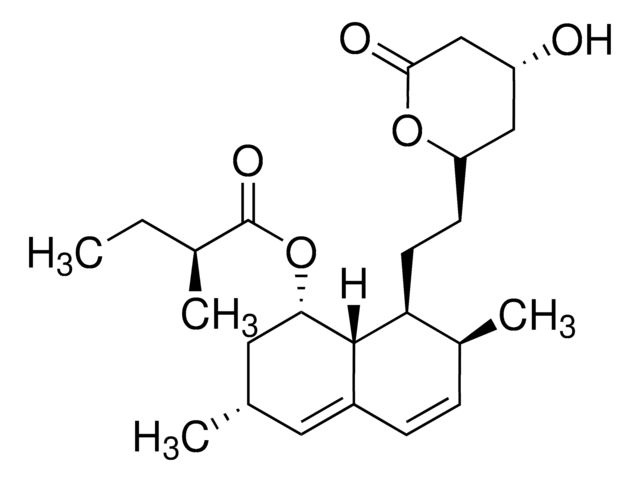
Aphidicolin, Ready Made Solution
from Nigrospora sphaerica
Synonyme(s) :
ICI 69653, NSC-234714
About This Item
Produits recommandés
Source biologique
Nigrospora sphaerica
Niveau de qualité
Pression de vapeur
0.55 hPa ( 20 °C)
Forme
liquid
Couleur
clear colorless
Solubilité
H2O: miscible (completely)
Spectre d'activité de l'antibiotique
neoplastics
viruses
Mode d’action
DNA synthesis | interferes
enzyme | inhibits
Température de stockage
−20°C
InChI
1S/C20H34O4/c1-17(11-21)15-4-3-13-9-14-10-19(13,7-8-20(14,24)12-22)18(15,2)6-5-16(17)23/h13-16,21-24H,3-12H2,1-2H3/t13-,14+,15-,16+,17-,18-,19-,20-/m0/s1
Clé InChI
NOFOAYPPHIUXJR-APNQCZIXSA-N
Description générale
Application
Actions biochimiques/physiologiques
Conditionnement
Forme physique
Autres remarques
Code de la classe de stockage
10 - Combustible liquids
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
188.6 °F - closed cup
Point d'éclair (°C)
87 °C - closed cup
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique