Alle Fotos(3)
Wichtige Dokumente
M8765
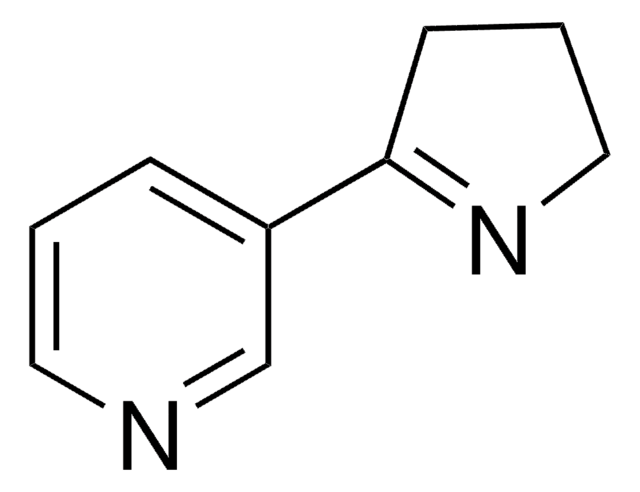
Myosmin
≥98%
Synonym(e):
3-(1-Pyrrolin-2-yl)-pyridin
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(3)
About This Item
Empirische Formel (Hill-System):
C9H10N2
CAS-Nummer:
Molekulargewicht:
146.19
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
≥98%
Form
solid
Lagertemp.
2-8°C
SMILES String
C1CN=C(C1)c2cccnc2
InChI
1S/C9H10N2/c1-3-8(7-10-5-1)9-4-2-6-11-9/h1,3,5,7H,2,4,6H2
InChIKey
DPNGWXJMIILTBS-UHFFFAOYSA-N
Angaben zum Gen
rat ... Chrna4(25590)
Verwandte Kategorien
Anwendung
Myosmine can be used as a reactant in thesynthesis of nicotinoid derivatives through N-nitrosation.
Tobacco alkaloid
Reactant for:
Nitrosation reactions
Peroxidation reaction with hydrogen peroxide
Reactant for:
Nitrosation reactions
Peroxidation reaction with hydrogen peroxide
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Trixie Ann Bartholomeusz et al.
Phytochemistry, 66(20), 2432-2440 (2005-09-06)
Nicotine or nornicotine enriched with stable isotopes in either the N'-methyl group or the pyrrolidine-N were fed to Nicotiana plumbaginifolia suspension cell cultures that do not form endogenous nicotine. The metabolism of these compounds was investigated by analysing the incorporation
J S LaKind et al.
Risk analysis : an official publication of the Society for Risk Analysis, 19(3), 375-390 (2000-04-15)
The ultimate goal of the research reported in this series of three articles is to derive distributions of doses of selected environmental tobacco smoke (ETS)-related chemicals for nonsmoking workers. This analysis uses data from the 16-City Study collected with personal
J Wilp et al.
Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association, 40(8), 1223-1228 (2002-06-18)
The tobacco alkaloid myosmine was detected in nut and nut products [J. Agric. Food Chem. 46 (1998) 2703]. Upon nitrosation, myosmine yields 4-hydroxy-1-(3-pyridyl)-1-butanone (HPB) and N-nitrosonornicotine (NNN) [J. Agric. Food Chem. 48 (2001) 392]. NNN is a strong oesophageal carcinogen
Stefan Tyroller et al.
Journal of agricultural and food chemistry, 50(17), 4909-4915 (2002-08-09)
Myosmine has been regarded as a specific tobacco alkaloid until investigations pointed out that nuts and nut products constitute a significant source of myosmine. In the present study it is shown that the occurrence of myosmine is widespread throughout a
Wei Li et al.
Scientific reports, 7(1), 12126-12126 (2017-09-25)
Leaf senescence in plants is a coordinated process that involves remobilization of nutrients from senescing leaves to sink tissues. The molecular events associated with nutrient remobilization are however not well understood. In this study the tobacco system with a source-sink
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.