914088
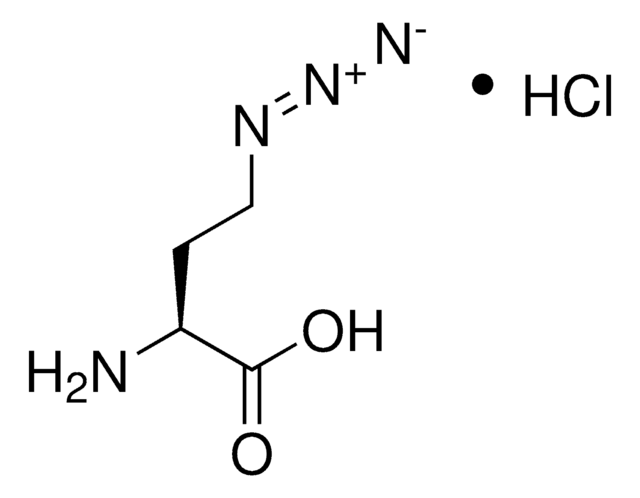
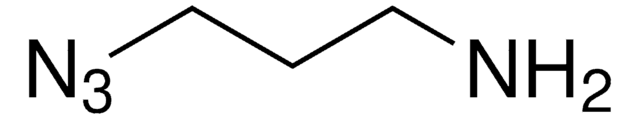
N6-((2-Azidoethoxy)carbonyl)-L-lysine hydrochloride
≥95%
Synonym(e):
(S)-2-amino-6-((2-azidoethoxy)carbonylamino)hexanoic acid hydrochloride, Clickable amino acid for bioconjugation, H-L-Lys(EO-N3)-OH HCl, Lysine-azide, UAA crosslinker
About This Item
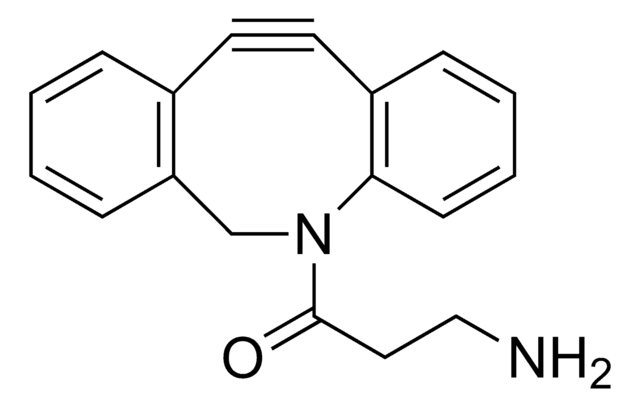
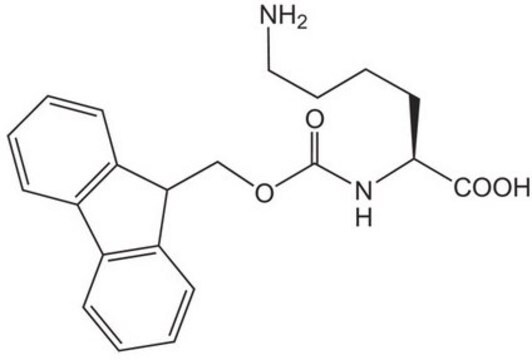
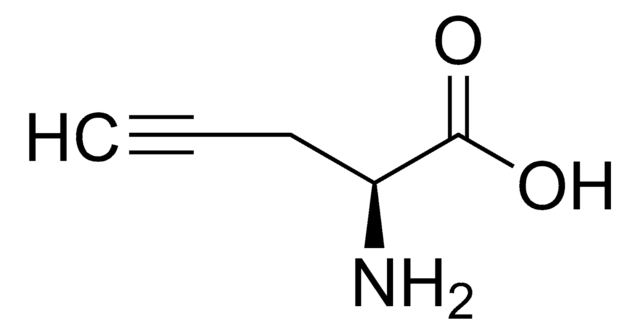
Empfohlene Produkte
Anwendung
Sonstige Hinweise
Semisynthesis of an Active Enzyme by Quantitative Click Ligation
A Robust and Quantitative Reporter System To Evaluate Noncanonical Amino Acid Incorporation in Yeast
An orthogonalized platform for genetic code expansion in both bacteria and eukaryotes
Ähnliches Produkt
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Self-react. C
Lagerklassenschlüssel
5.2 - Organic peroxides and self-reacting hazardous materials
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.