900856
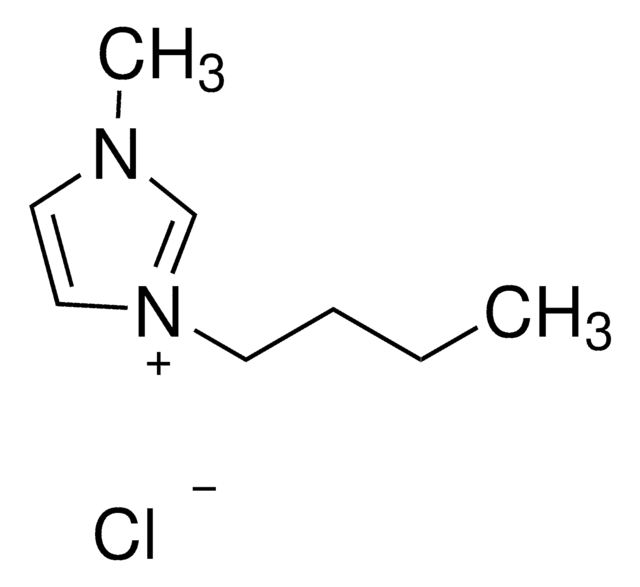
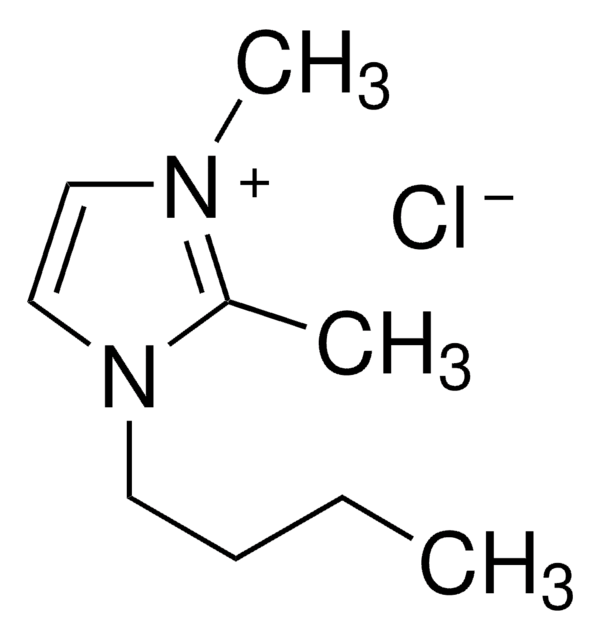
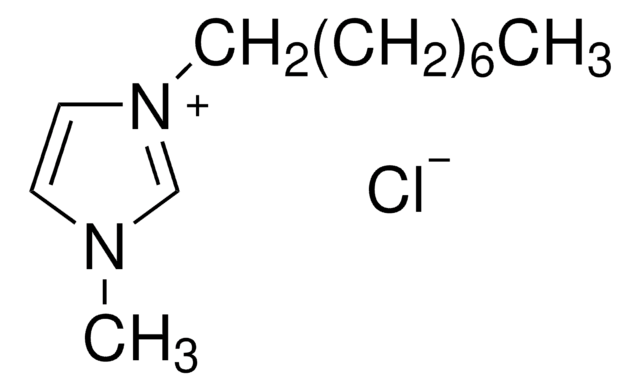
1-Butyl-3-Methylimidazolium-Chlorid
≥99%
Synonym(e):
BMIMCl
About This Item
Empfohlene Produkte
Qualitätsniveau
Assay
≥99%
Form
solid
Grünere Alternativprodukt-Eigenschaften
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp (Schmelzpunkt)
~70 °C
Anwendung(en)
battery manufacturing
Grünere Alternativprodukt-Kategorie
, Aligned
SMILES String
[Cl-].CCCCn1cc[n+](C)c1
InChI
1S/C8H15N2.ClH/c1-3-4-5-10-7-6-9(2)8-10;/h6-8H,3-5H2,1-2H3;1H/q+1;/p-1
InChIKey
FHDQNOXQSTVAIC-UHFFFAOYSA-M
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
Click here for more information.
Anwendung
Vorsicht
Ähnliches Produkt
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Acute Tox. 3 Oral - Aquatic Chronic 2 - Eye Irrit. 2 - Skin Irrit. 2
Lagerklassenschlüssel
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flammpunkt (°F)
377.6 °F - (External MSDS)
Flammpunkt (°C)
192 °C - (External MSDS)
Hier finden Sie alle aktuellen Versionen:
Analysenzertifikate (COA)
Die passende Version wird nicht angezeigt?
Wenn Sie eine bestimmte Version benötigen, können Sie anhand der Lot- oder Chargennummer nach einem spezifischen Zertifikat suchen.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Artikel
Dr. Sun reviews the recent advances in solid-state rechargeable batteries and cover the fundamentals of solid electrolytes in solid-state batteries, the theory of ion conduction, and the structures and electrochemical processes of solid-state Li batteries.
Here, we present a short review of ionic liquid electrolytes used in state-of-the-art rechargeable batteries including high performance and low-cost aluminum batteries, non-flammable Li-based batteries, and high-cycling and stable dual-graphite batteries. We also outline the key issues explored so as to identify the future direction of IL development.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.