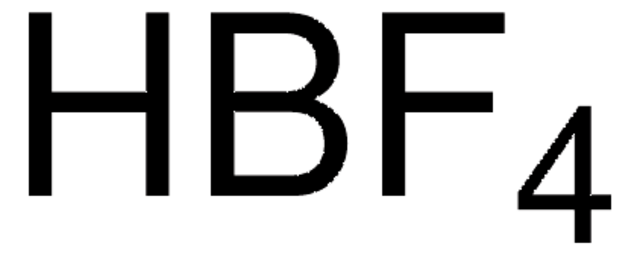745537
4-(Acetylamino)-2,2,6,6-tetramethyl-1-oxo-piperidinium tetrafluoroborate
97% (HPLC)
Synonym(e):
4-Acetamido-2,2,6,6-tetramethyl-1-oxopiperidinium tetrafluoroborate, Bobbitt′s Salt
About This Item
Empfohlene Produkte
Qualitätsniveau
Assay
97% (HPLC)
Form
solid
Eignung der Reaktion
reagent type: oxidant
mp (Schmelzpunkt)
191-197 °C (decomposition)
Funktionelle Gruppe
amide
Lagertemp.
2-8°C
SMILES String
F[B-](F)(F)F.CC(=O)NC1CC(C)(C)[N+](=O)C(C)(C)C1
InChI
1S/C11H20N2O2.BF4/c1-8(14)12-9-6-10(2,3)13(15)11(4,5)7-9;2-1(3,4)5/h9H,6-7H2,1-5H3;/q;-1/p+1
InChIKey
HTMHEICBCHCWAU-UHFFFAOYSA-O
Allgemeine Beschreibung
Anwendung
- Oxidation of alcohols to their concomitant aldehyde, ketone or carboxylic acid.
- Conversion of aldehydes to hexafluoroisopropyl (HFIP) esters via oxidative esterification.,·
- Deprotection of allyl ethers to corresponding aldehydes.
- Preparation of α,β-unsaturated ketones by dehydrogenation of perfluoroalkyl ketones.
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Verwandter Inhalt
Dr. James Bobbitt has been developing the chemistry of oxoammonium salts, such as Bobbitt's Salt (Product 745537), for 30 years. He discovered the oxoaommonium chemistry by accident in 1985 and found that it had been started by a fine Russian chemist called Valery Golubev. At the University of Connecticut, he has had major collaborations with Professor Christian Bruckner, Professor William Bailey, and Professor Nicholas Leadbeater. He does most of his own experimental work, much of which has been published. Even though he is into his mid-80's, he continues to work on several chemistry projects as an Emeritus Professor at UConn.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.