Alle Fotos(1)
Wichtige Dokumente
671576
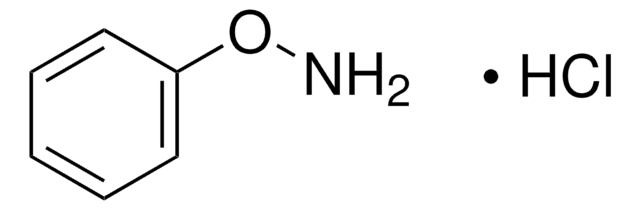
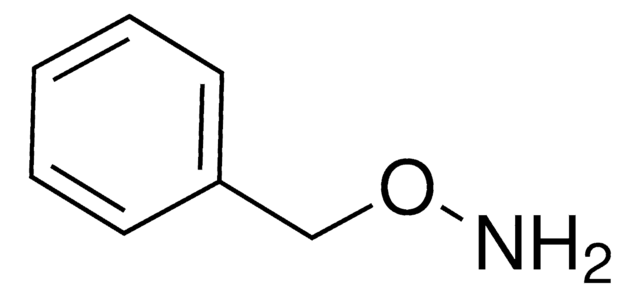
N-Phenylhydroxylamin
≥95.0%
Synonym(e):
N-Hydroxyphenylamin
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C6H7NO
CAS-Nummer:
Molekulargewicht:
109.13
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
≥95.0%
Form
solid
mp (Schmelzpunkt)
80-84 °C
Lagertemp.
−20°C
SMILES String
ONc1ccccc1
InChI
1S/C6H7NO/c8-7-6-4-2-1-3-5-6/h1-5,7-8H
InChIKey
CKRZKMFTZCFYGB-UHFFFAOYSA-N
Anwendung
N-Phenylhydroxylamine can be used as a starting material for the synthesis of:
- 2-alkylindoles by treating with aliphatic terminal alkynes using gold catalyst via sequential 3,3-rearrangements and cyclodehydrations.
- Isoxazolidines by reacting with aldehydes and α, β-unsaturated aldehydes via a three-component one-pot catalytic reaction.
- Tetrahydro-1,2-oxazines by treating with an aldehyde and cyclopropane via homo 3+2 dipolar cycloaddition reaction.
Signalwort
Danger
H-Sätze
P-Sätze
Gefahreneinstufungen
Acute Tox. 3 Oral
Lagerklassenschlüssel
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Au-catalyzed synthesis of 2-alkylindoles from N-arylhydroxylamines and terminal alkynes
Wang Y, et al.
Chemical Communications (Cambridge, England), 47(27), 7815-7817 (2011)
T P Bradshaw et al.
Free radical biology & medicine, 18(2), 279-285 (1995-02-01)
Previous studies have shown that incubation of rat red blood cells in vitro with phenylhydroxylamine (50-300 microM) induces rapid splenic sequestration of the red cells on reintroduction to isologous rats. EPR and the spin trapping agent, 5,5-dimethyl-1-pyrroline-N-oxide (DMPO), were utilized
Nilanjana Chowdhury et al.
Bioorganic & medicinal chemistry letters, 20(18), 5414-5417 (2010-08-21)
Photoinduced homolytic fission of nitrogen-oxygen bond in N,O-diacyl-4-benzoyl-N-phenylhydroxylamines using 310 nm UV light for 10 min produced acylaminyl and acyloxy radicals, which resulted in single strand cleavage of DNA at pH 7.0. Further the DNA cleaving ability of N,O-diacyl-4-benzoyl-N-phenylhydroxylamines found
A simple one-pot, three-component, catalytic, highly enantioselective isoxazolidine synthesis
Rios R, et al.
Tetrahedron Letters, 48(32), 5701-5705 (2007)
Christine S Olver et al.
Blood coagulation & fibrinolysis : an international journal in haemostasis and thrombosis, 24(3), 273-278 (2012-12-12)
Carboxyheme and metheme states modulate hemostasis in humans and other species. Further, carbon monoxide and/or nitric oxide production increase in inflammatory disorders involving the gastrointestinal tract, with associated hypercoagulability or hypocoagulability. In particular, the horse suffers both thrombotic or coagulopathic
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.