560006
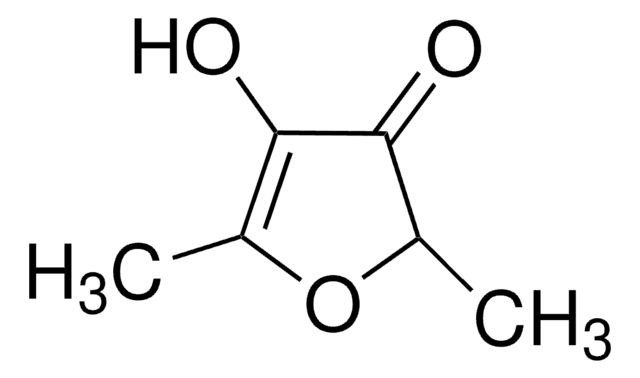
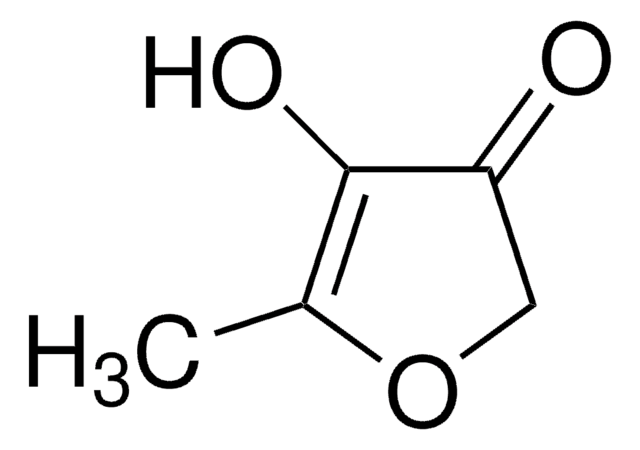
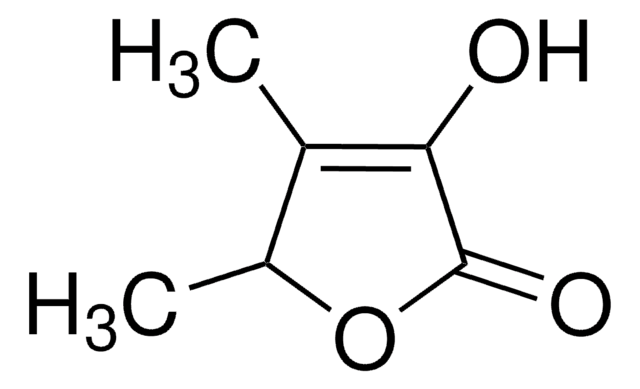
4-Hydroxy-5-methyl-3-furanon
97%
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Lineare Formel:
C4OH2OCH3OH
CAS-Nummer:
Molekulargewicht:
114.10
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Assay
97%
mp (Schmelzpunkt)
129-133 °C (lit.)
SMILES String
CC1=C(O)C(=O)CO1
InChI
1S/C5H6O3/c1-3-5(7)4(6)2-8-3/h7H,2H2,1H3
InChIKey
DLVYTANECMRFGX-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Anwendung
4-Hydroxy-5-methyl-3-furanone may be used in oxidoreductase assay for the evaluation of oxidoreductase activity.
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Highly effective inhibition of biofilm formation by the first metagenome-derived AI-2 quenching enzyme.
Weiland-Brauer N, et al.
Frontiers in Microbiology, 7 (2016)
Reinvestigation of the reaction between 2-furancarboxaldehyde and 4-hydroxy-5-methyl-3(2H)-furanone.
A Ravagli et al.
Journal of agricultural and food chemistry, 47(12), 4962-4969 (1999-12-22)
The reaction between 2-furancarboxaldehyde and 4-hydroxy-5-methyl-3(2H)-furanone was reinvestigated as a part of a systematic study on low molecular weight colored compounds from the Maillard reaction. In acetic acid/piperidine, besides 2-(2-furanylmethylene)-4-hydroxy-5-methyl-3(2H)-furanone (1) and 5-[2-(2-furanyl)ethenyl]-2-(2-furanylmethylene)-4-hydroxy-5-methyl -3( 2H)-furanone (2), four novel compounds, 15a
Tobias Hauck et al.
Journal of agricultural and food chemistry, 51(5), 1410-1414 (2003-02-20)
Formation of the flavor compound and precursor 4-hydroxy-5-methyl-3[2H]-furanone (HMF, norfuraneol) was demonstrated in cytosolic protein extracts obtained from Zygosaccharomyces rouxii after incubation with a number of carbohydrate phosphates. 4-Hydroxy-5-methyl-3[2H]-furanone was produced from d-fructose-1,6-diphosphate, d-fructose-6-phosphate, d-glucose-6-phosphate, 6-phosphogluconate, d-ribose-5-phosphate, and d-ribulose-1,5-diphosphate. Enzyme
F B Whitfield et al.
Journal of agricultural and food chemistry, 47(4), 1626-1634 (1999-11-24)
Reaction of 4-hydroxy-5-methyl-3(2H)-furanone (HMF) with cysteine or hydrogen sulfide at pH 4.5 for 60 min at 140 degrees C produced complex mixtures of volatile compounds, the majority of which contained sulfur. Sixty-nine compounds were identified, some tentatively, by GC/MS. These
H J Kim et al.
Advances in experimental medicine and biology, 434, 91-99 (1998-05-23)
The chemometric principle was used to derive a guideline for obtaining a simple "yes or no" answer about the sterility of food particulates heated at aseptic processing temperatures. A quadratic temperature pulse model was used to estimate bacterial destruction from
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.