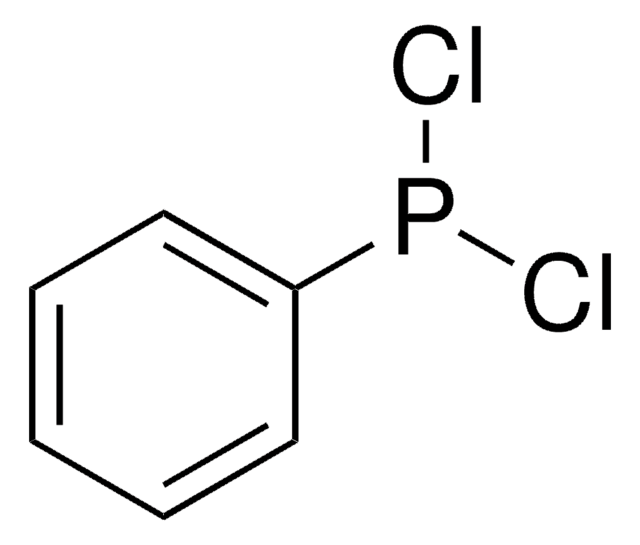
265004
(4R,5R)-2,2-Dimethyl-α,α,α′,α′-tetraphenyldioxolan-4,5-dimethanol
97%
Synonym(e):
(−)-2,3-O-Isopropyliden-1,1,4,4-tetraphenyl-L-threitol, (−)-trans-α,α′-(2,2-Dimethyl-1,3-dioxolan-4,5-diyl)-bis(diphenylmethanol), (4R,5R)-4,5-Bis-(diphenylhydroxymethyl)-2,2-dimethyldioxolan, 1,1,4,4-Tetraphenyl-2,3-O-isopropyliden-L-threitol, TADDOL
About This Item
Empfohlene Produkte
Qualitätsniveau
Assay
97%
Optische Aktivität
[α]19/D −62.6°, c = 1 in chloroform
mp (Schmelzpunkt)
193-195 °C (lit.)
SMILES String
CC1(C)O[C@H]([C@@H](O1)C(O)(c2ccccc2)c3ccccc3)C(O)(c4ccccc4)c5ccccc5
InChI
1S/C31H30O4/c1-29(2)34-27(30(32,23-15-7-3-8-16-23)24-17-9-4-10-18-24)28(35-29)31(33,25-19-11-5-12-20-25)26-21-13-6-14-22-26/h3-22,27-28,32-33H,1-2H3/t27-,28-/m1/s1
InChIKey
OWVIRVJQDVCGQX-VSGBNLITSA-N
Anwendung
Catalyst involved in synthesis of cyclopropylamines via addition reactions of Grignard reagents to amides
Reactant or reagent involved in:
- Enantioswitching of catalytic asymmetric hydroboration
- Synthesis of derivative ligands for asymmetric hydroformylation of alkenes
- Amide-directed catalytic asymmetric hydroboration of trisubstituted alkenes
- Addition of deactivated alkyl Grignard reagents to aldehydes
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Hier finden Sie alle aktuellen Versionen:
Analysenzertifikate (COA)
Die passende Version wird nicht angezeigt?
Wenn Sie eine bestimmte Version benötigen, können Sie anhand der Lot- oder Chargennummer nach einem spezifischen Zertifikat suchen.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Artikel
The chiral auxiliaries TADDOLs (α,α,α,α-tetraaryl-1,3-dioxolane-4,5- dimethanols) developed by Seebach's group have found numerous applications in asymmetric synthesis ranging from utilization as stoichiometric chiral reagents or in Lewis acid mediated reactions, to roles in catalytic hydrogenation and stereoregular metathesis polymerization.
Apart from numerous examples using TADDOLs in metal-catalyzed asymmetric reactions, Rawal recently reported that TADDOLs could be used as Brønsted acid organocatalysts in highly stereoselective hetero-Diels–Alder reactions.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.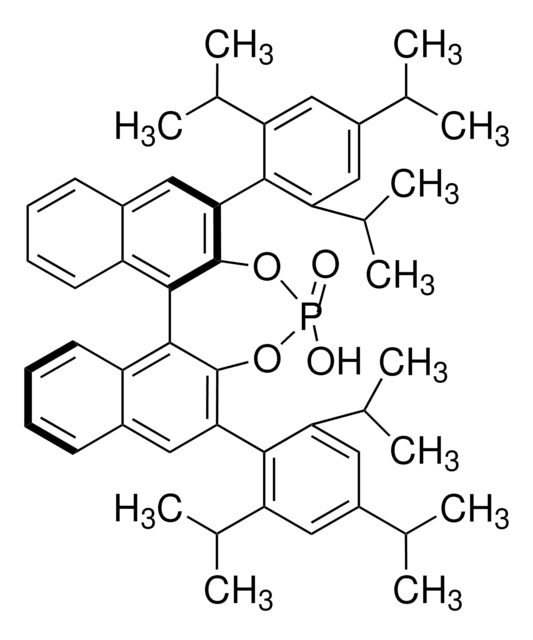

![[Ir(dFCF3ppy)2-(5,5’-dCF3bpy)]PF6 ≥95%](/deepweb/assets/sigmaaldrich/product/structures/422/901/e00f3148-fb86-4f94-9e79-21d064c3f327/640/e00f3148-fb86-4f94-9e79-21d064c3f327.png)