Wichtige Dokumente
241636
2,2′-Bithiophen
99%
Synonym(e):
2,2′-Bithienyl, 2,2′-Dithienyl
About This Item
Empfohlene Produkte
Assay
99%
bp
260 °C (lit.)
mp (Schmelzpunkt)
32-33 °C (lit.)
SMILES String
c1csc(c1)-c2cccs2
InChI
1S/C8H6S2/c1-3-7(9-5-1)8-4-2-6-10-8/h1-6H
InChIKey
OHZAHWOAMVVGEL-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
Anwendung
Lagerklassenschlüssel
10 - Combustible liquids
WGK
WGK 3
Flammpunkt (°F)
230.0 °F - closed cup
Flammpunkt (°C)
110 °C - closed cup
Persönliche Schutzausrüstung
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Artikel
From Form to Function: Molding Porous Materials in Three Dimensions by Colloidal Crystal Templating
Professor Rivnay (Northwestern University, USA) discusses using organic mixed conductors as an alternative to efficiently bridge the ionic world of biology with contemporary microelectronics.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.
![Thieno[3,2-b]thiophen 95%](/deepweb/assets/sigmaaldrich/product/structures/353/609/429fd4bf-e217-4371-80a3-9e5a4d88908b/640/429fd4bf-e217-4371-80a3-9e5a4d88908b.png)
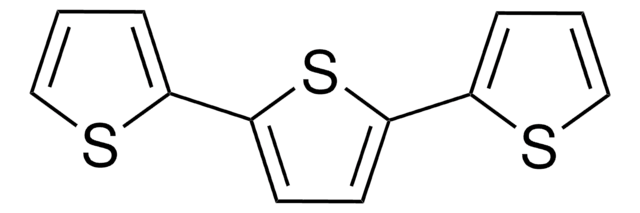
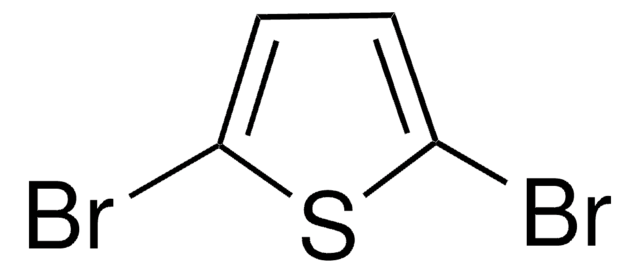
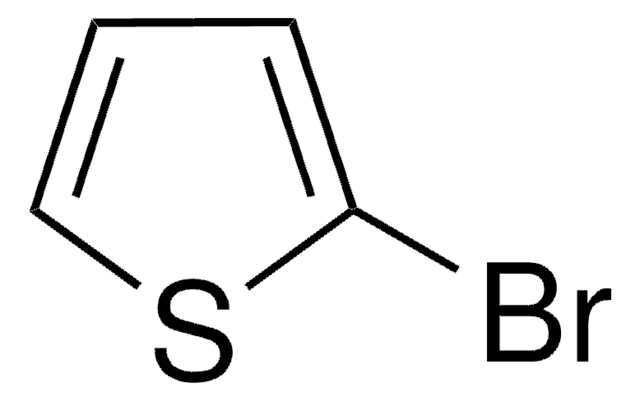
![2,5-Bis(trimethylstannyl)-thieno[3,2-b]thiophene 97%](/deepweb/assets/sigmaaldrich/product/structures/126/532/26557e94-858e-4c96-90de-ca88d84a8727/640/26557e94-858e-4c96-90de-ca88d84a8727.png)
![Benzo[1,2-b:4,5-b′]dithiophene-4,8-dione 97%](/deepweb/assets/sigmaaldrich/product/structures/418/544/b7faac0b-ad09-4b42-a9fa-aeb38017a39e/640/b7faac0b-ad09-4b42-a9fa-aeb38017a39e.png)