143685
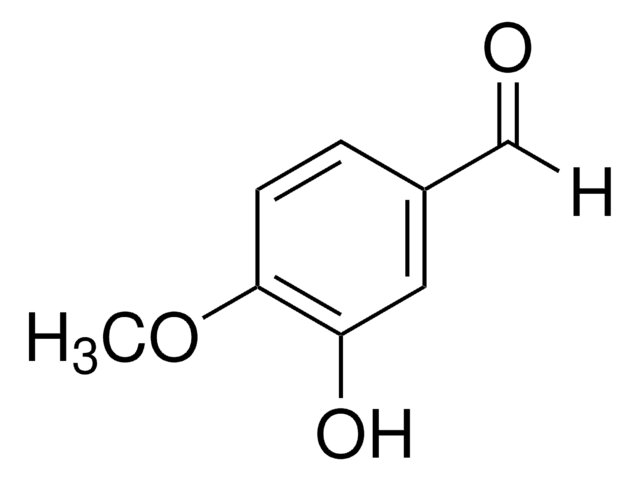
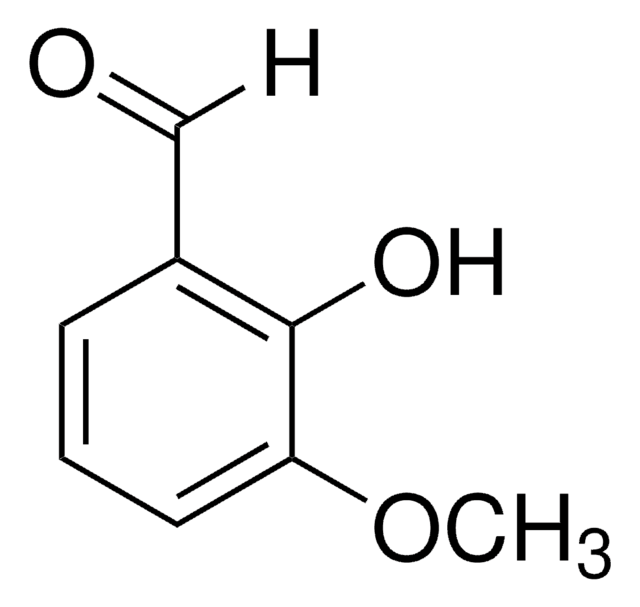
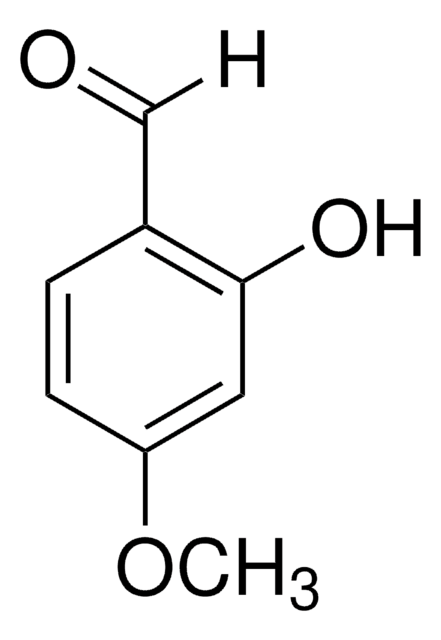
Isovanillin
99%
Synonym(e):
3-Hydroxy-4-methoxy-benzaldehyd, 3-Hydroxyanisaldehyd
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Lineare Formel:
HOC6H3(OCH3)CHO
CAS-Nummer:
Molekulargewicht:
152.15
Beilstein:
1073021
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
99%
bp
179 °C/15 mmHg (lit.)
mp (Schmelzpunkt)
113-115 °C (lit.)
Funktionelle Gruppe
aldehyde
SMILES String
[H]C(=O)c1ccc(OC)c(O)c1
InChI
1S/C8H8O3/c1-11-8-3-2-6(5-9)4-7(8)10/h2-5,10H,1H3
InChIKey
JVTZFYYHCGSXJV-UHFFFAOYSA-N
Verwandte Kategorien
Allgemeine Beschreibung
3-Hydroxy-4-methoxybenzaldehyde on condensation with furan-2-carboxylic acid hydrazide and thiophene-2-carboxylic acid hydrazide yields Schiff-bases. It undergoes condensation reaction with1-azabicyclo[2.2.2]octan-3-one to give (Z)-2-(3-hydroxy-4-methoxybenzylidene)-1-azabicyclo[2.2.2]octan-3-one.
Anwendung
3-Hydroxy-4-methoxybenzaldehyde was used as starting reagent during the two-step stereoselective synthesis of the anticancer drug (Z)-combretastatin A-4 and glycitein synthesis.
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
>212.0 °F
Flammpunkt (°C)
> 100 °C
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Caroline Lang'at-Thoruwa et al.
Journal of natural products, 66(1), 149-151 (2003-01-25)
4-Methoxyresorcinol (3) was synthesized as the precursor for glycitein (6) synthesis by the oxidation of 3-hydroxy-4-methoxybenzaldehyde (1) to the aryl formate with H2O2 and a catalytic amount of SeO2. Glycitein (6) was synthesized by cyclization of 2,4,4'-trihydroxy-5-methoxydeoxybenzoin (5) with N,N-dimethylformamide
K Gaukroger et al.
The Journal of organic chemistry, 66(24), 8135-8138 (2001-11-28)
A high-yielding, two-step stereoselective synthesis of the anticancer drug (Z)-combretastatin A-4 (1) has been devised. The method uses the Perkin condensation of 3,4,5-trimethoxyphenylacetic acid and 3-hydroxy-4-methoxybenzaldehyde followed by decarboxylation of the cinnamic acid intermediate using copper and quinoline. The iodine-catalyzed
Vijayakumar N Sonar et al.
Acta crystallographica. Section C, Crystal structure communications, 59(Pt 11), o647-o649 (2003-11-08)
Crystals of the title compound, C(15)H(17)NO(3), were obtained from a condensation reaction of 3-hydroxy-4-methoxybenzaldehyde with 1-azabicyclo[2.2.2]octan-3-one and subsequent crystallization of the product from methanol. The title compound, containing a double bond that connects the azabicyclic ring system to the 3-hydroxy-4-methoxybenzylidene
Riyadh M Ahmed et al.
TheScientificWorldJournal, 2013, 754868-754868 (2013-09-13)
New monomeric cobalt and cadmium complexes with Schiff-bases, namely, N'-[(E)-(3-hydroxy-4-methoxyphenyl)methylidene]furan-2-carbohydrazide (L¹) and N'-[(E)-(3-hydroxy-4-methoxyphenyl)methylidene]thiophene-2-carbohydrazide (L²) are reported. Schiff-base ligands L¹ and L² were derived from condensation of 3-hydroxy-4-methoxybenzaldehyde (iso-vanillin) with furan-2-carboxylic acid hydrazide and thiophene-2-carboxylic acid hydrazide, respectively. Complexes of the
Michael D Markey et al.
Organic letters, 9(17), 3255-3257 (2007-07-31)
The first total synthesis of santiagonamine (1) is achieved in 12 steps from isovanillin. A palladium-catalyzed Ullmann cross-coupling reaction and a photocyclization are the key steps in the synthesis.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.