Use of Iron Oxide Incorporated Conjugated Polymer Nanoparticles for Bioimaging
Dr. Dermott O'Callaghan
Head of Product Development at Stream Bio
Iron Oxide Incorporated Conjugated Polymer Nanoparticles: Exceptional Brightness & Versatility in Fluorescent Labeling
Iron oxide incorporated conjugated polymer nanoparticles, also known as Conjugated Polymer Nanoparticles (CPNs), are highly fluorescent nanoparticles comprised of a semiconductor light emitting polymer (LEP) core encapsulated within a biocompatible surfactant. These innovative molecular bioimaging probes have fluorescent properties significantly exceeding those of other labeling agents. Their intense brightness is a result of their exceptional extinction coefficients and outstanding photo-, thermo- and chemical stability.1 Furthermore, no toxicity has been exhibited by CPNs, making them readily compatible with live cell systems.2,3 The CPN core also contains an iron oxide component, making the nanoparticles amenable to magnetic manipulation and, thus, ideal multi-modal MRI imaging agents. Table 1 shows a complete list of CPN properties.
The CPN range covers the fluorescence emission wavelengths from 420 nm to 680 nm in the visible spectrum. Due to this spectral coverage, CPNs can find a multitude of applications in flow cytometry, immunohistochemistry, ELISA, and in lateral flow devices, with the range matching standard filter sets and laser lines. These techniques are enabled by surface-accessible carboxyl groups, which allow the conjugation of a wide range of molecules, including antibodies, proteins, streptavidin, and nucleic acids (Figure 1).
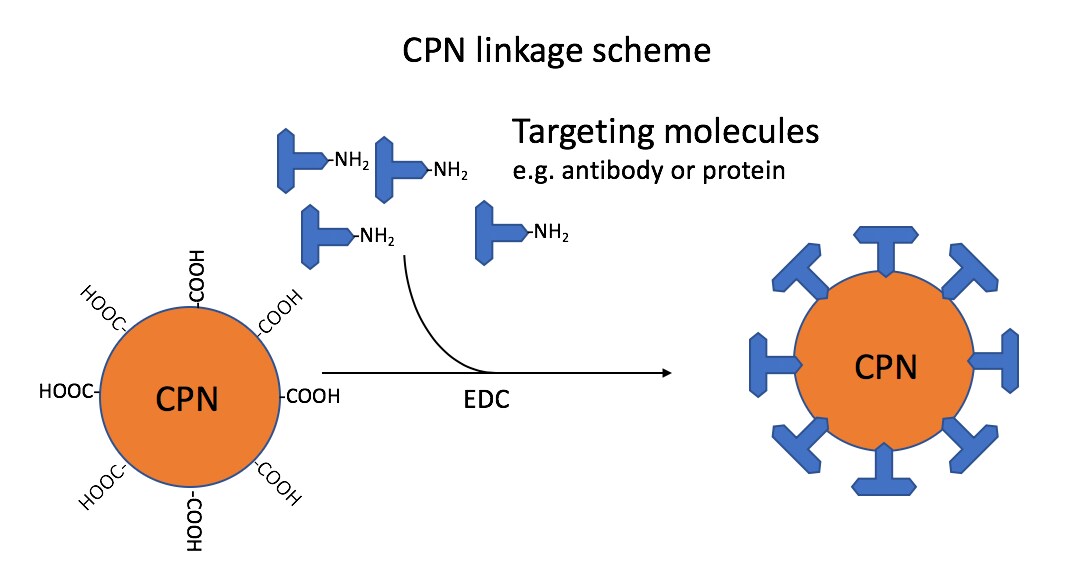
Figure 1.Linkage scheme: CPNs linked via carboxyl groups to amino groups on targeting molecules, such as antibodies. This reaction is mediated by the use of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC). The ratio used in the standard linkage protocol would attach approximately 40 antibody IgG to each CPN.
Characteristics of CPNs
Structural Properties
The LEP encapsulated within the biocompatible surfactant shell makes for a highly hydrophilic structure that permits the formation of water-soluble micelles with distinct sizes (Figure 2). This 'core-shell' structure (Figure 3) provides a suitable site upon which functionalizing molecules (such as streptavidin, antibodies, targeting proteins or nucleic acids) can be conjugated through a variety of surface chemistries.
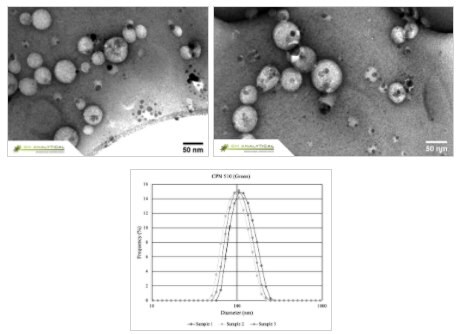
Figure 2.CPNs display excellent uniformity in size and shape, as shown in the Transmission Electron Microscopy (TEM) above, and Dynamic Light Scattering (DLS) below.
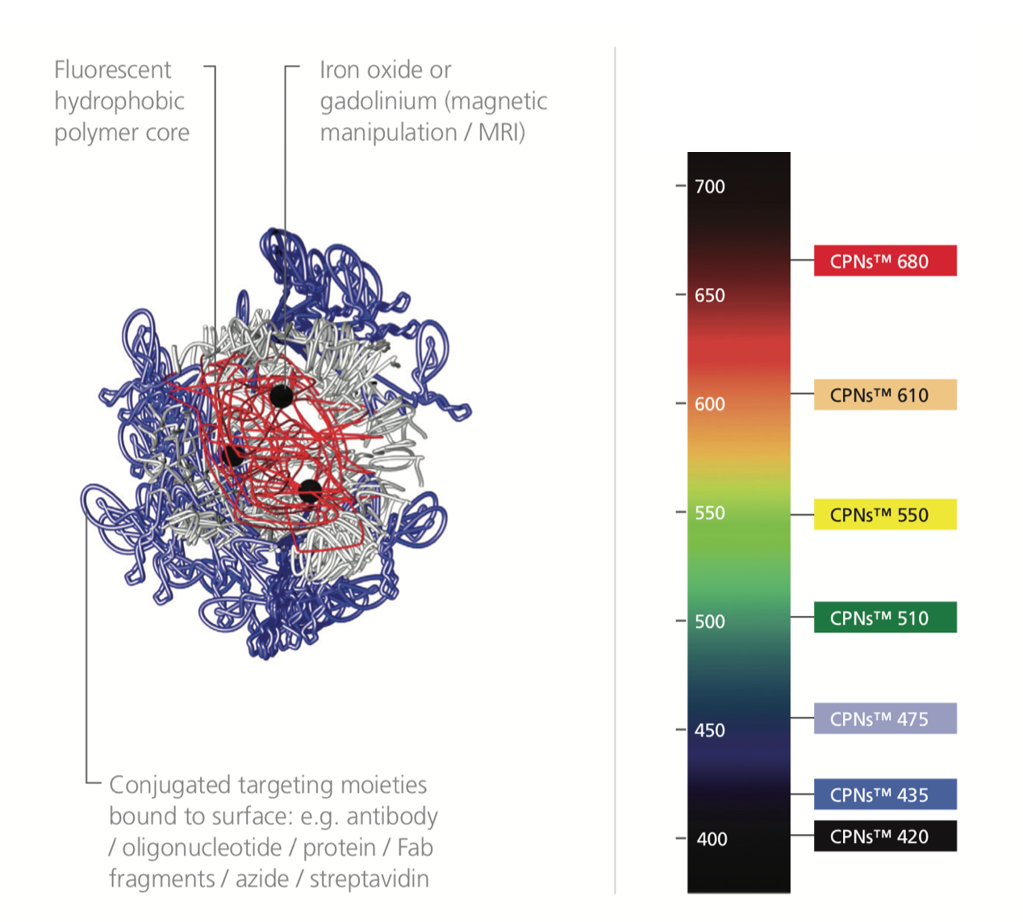
Figure 3.CPN structure alongside a light spectrum labeled with the range of CPNs available. CPNs absorb with a notable Stokes shift, larger than many organic dyes.
The integration of iron oxide within the core allows manipulation of the attached molecules or cells using magnetism (Figure 4), facilitating target enrichment and purification. Their iron oxide component makes CPNs into agents capable of multi-modal imaging, as they can be imaged both by fluorescence and using Magnetic Resonance Imaging (MRI).2,15 The magnetic properties can also be exploited in binding and plate-based assays, where they increase the assay signal and detection window, allowing for greater sensitivity when detecting low-concentration biomarkers.
A)

B)
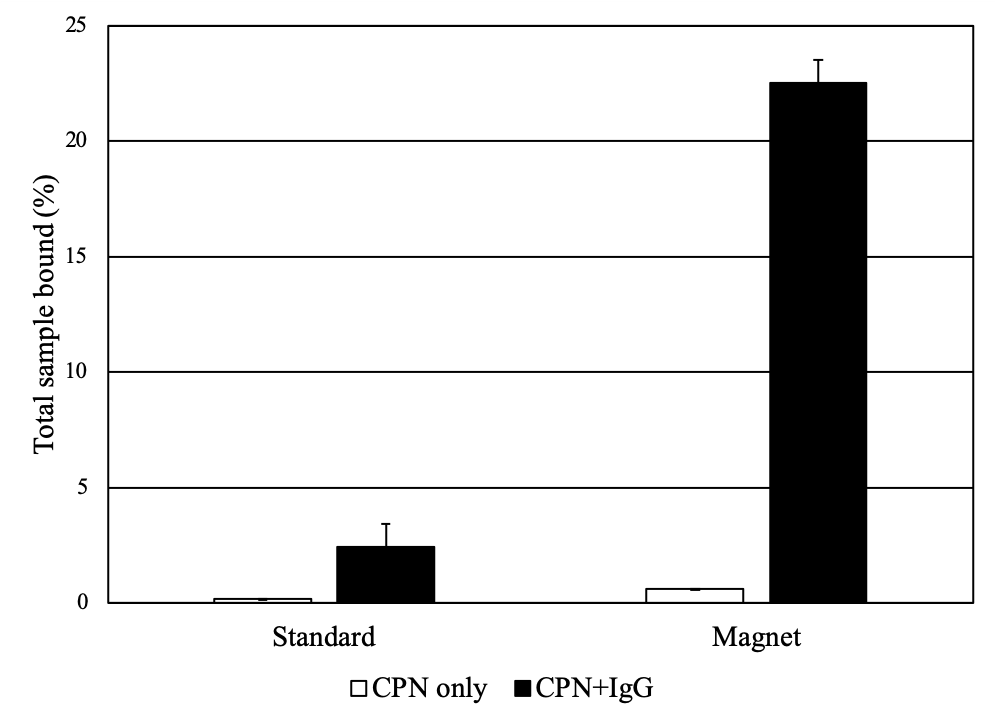
Figure 4.A.Solutions of CPNs under UV illumination and magnetic fields, outlining their dual modality for magnetism and fluorescence. (Edited and reprinted from Howes et al.)2 B) CPNs increase propensity for binding in a plate-based IgG binding assay. CPNs either unlinked or linked to IgG were incubated on plates coated with protein A/G to measure binding events. CPNs linked to IgG display a far stronger assay signal when a magnet is applied, as CPNs are pulled down towards the bottom of the plate, locally increasing their concentration at the site of the target.
Optical Properties
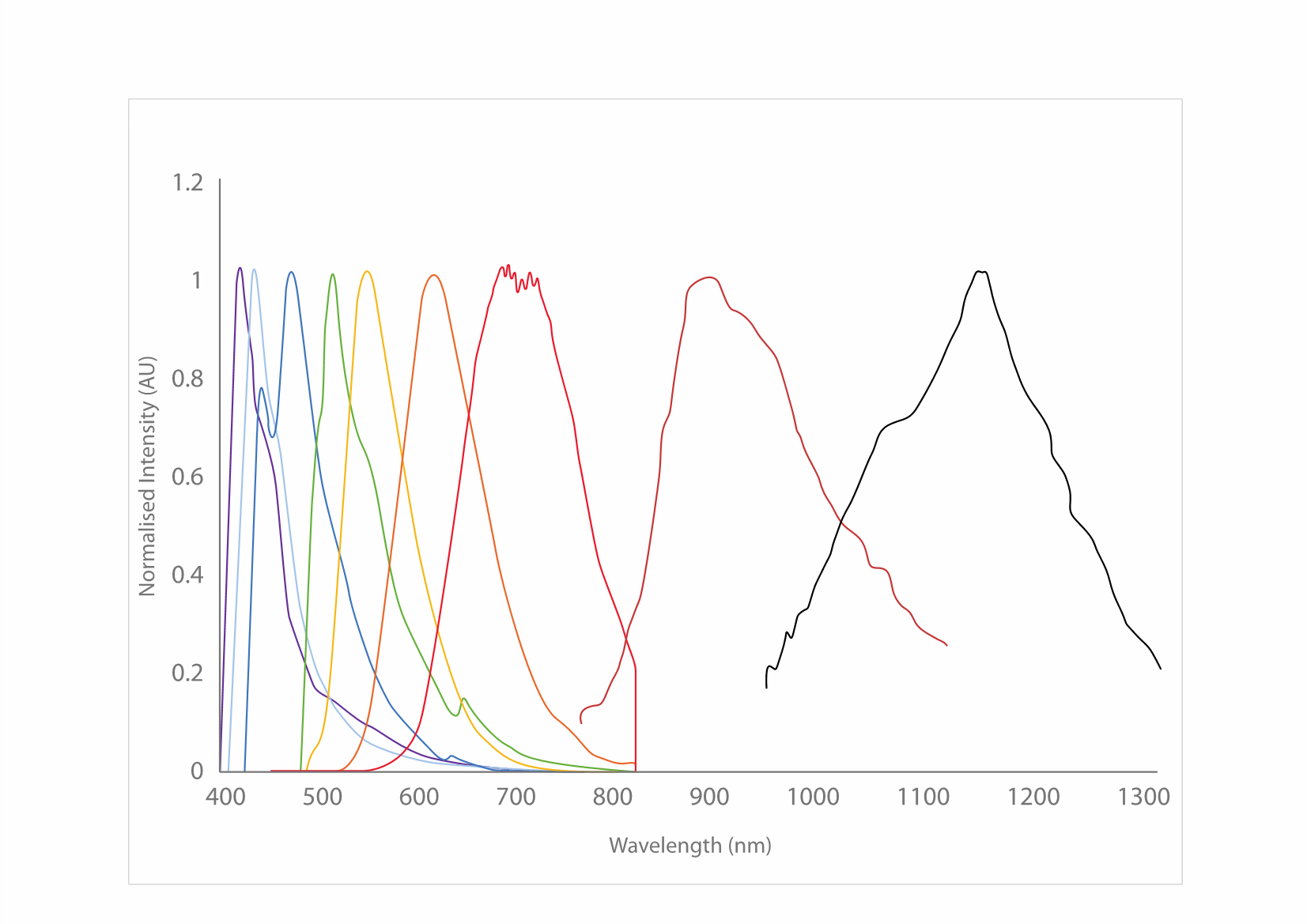
With their intense brightness and high degree of photostability, CPNs can improve a variety of imaging and diagnostics techniques. The color of light emitted by CPNs depends on the specific polymer core, with products offered in a range of fluorescence emission wavelengths. They are currently available in seven visible emission wavelengths, with each product line displaying discrete emission peaks at 420 nm, 435 nm, 475 nm, 510 nm, 550 nm, 610 nm, or 680 nm, with emission peaks at 900 nm and 1130 nm (Figure 5). Conjugated polymer nanoparticles have been reported to be 175x brighter than quantum dots, and 1400x brighter than AF488-dex in cells.3,14
A)
B)
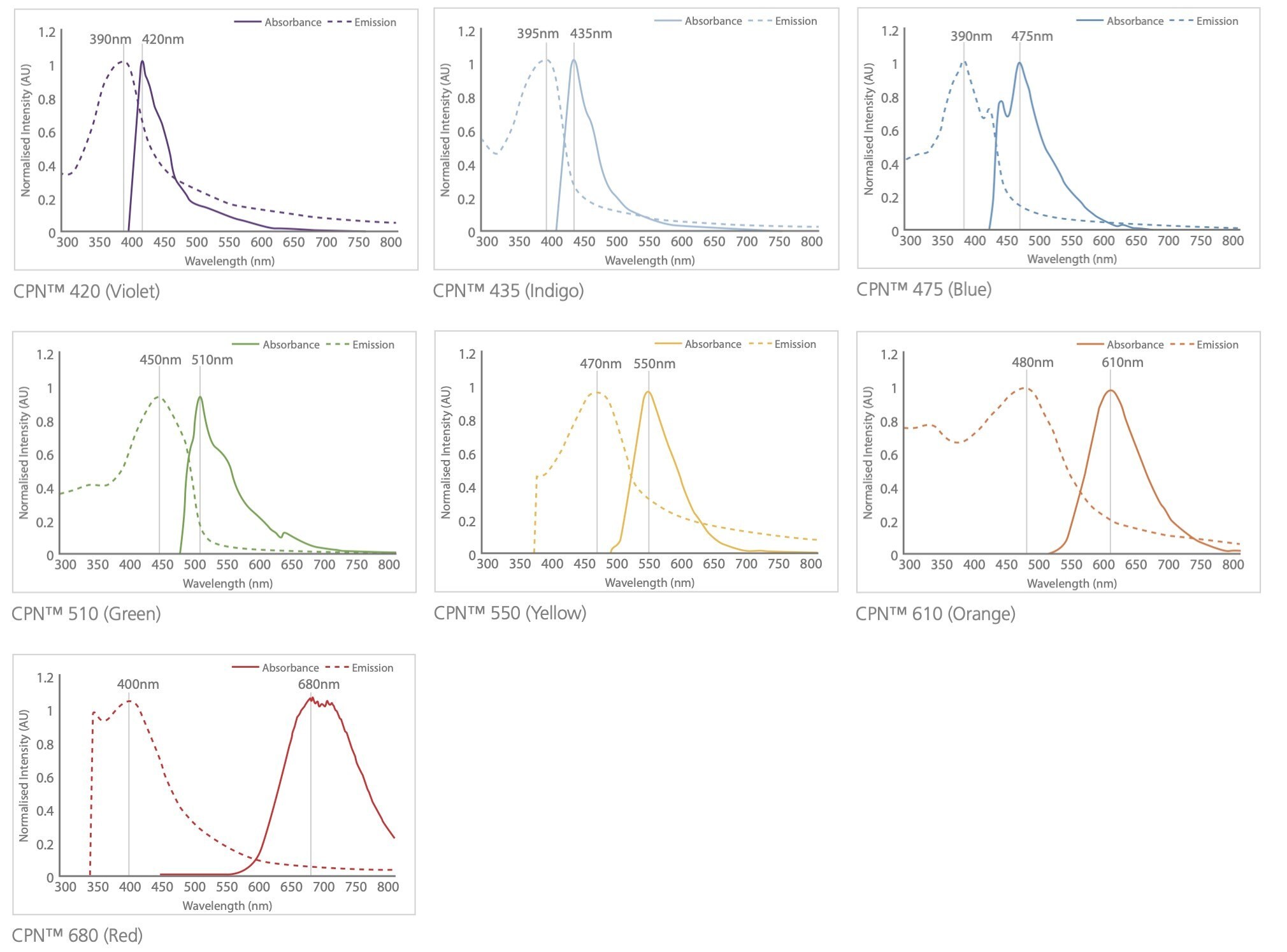
Figure 5.Excitation and emission spectra for the CPN range, covering the visible and NIR spectra.
In both conjugated and non-conjugated forms, CPNs retain their fluorescence for up to 24 months, even when stored under ambient temperature and lighting conditions (Figure 6).

Figure 6.CPNs remain photostable for up to 24 months, retaining their immense fluorescence over a long period of storage.
Enhanced Biofunctionalization
CPNs can label targeted cells through endocytosis or by linkage to specific targeting moieties, such as streptavidin, antibodies or receptor ligand proteins, making them suitable for a broad range of binding and targeting applications.
Coupling of a CPN to a targeting moiety involves EDC chemistry (N-ethyl-N'-dimethylaminopropyl-carbodiimide) to link amine groups (-NH2) on the protein to the exposed carboxyl groups on the surface of the CPN (-COOH). Other surface formulations are also available, such as thiol and azide for click chemistry. The brightness of CPNs ensures that detection is highly sensitive, with detection of single molecules in flow cytometry and immunocyto/histochemistry.16 This allows the study of individual proteins in samples and cells.
Application Versatility
The bright fluorescence, stability, and multi-modality of CPNs make them highly functional and applicable across numerous life sciences applications, showing the ability to enhance a variety of techniques (Figure 7).
- Fluorescence Microscopy (high content screening, live cell tracking and 3-D cell imaging)9,16
- Immunohistochemistry and Immunocytochemistry16
- Flow Cytometry5,9,17
- Rapid Diagnostic Tests (RDTs) 12,13
- Tissue Imaging6–8
- 2-Photon Imaging
- NIR Imaging10,11,15
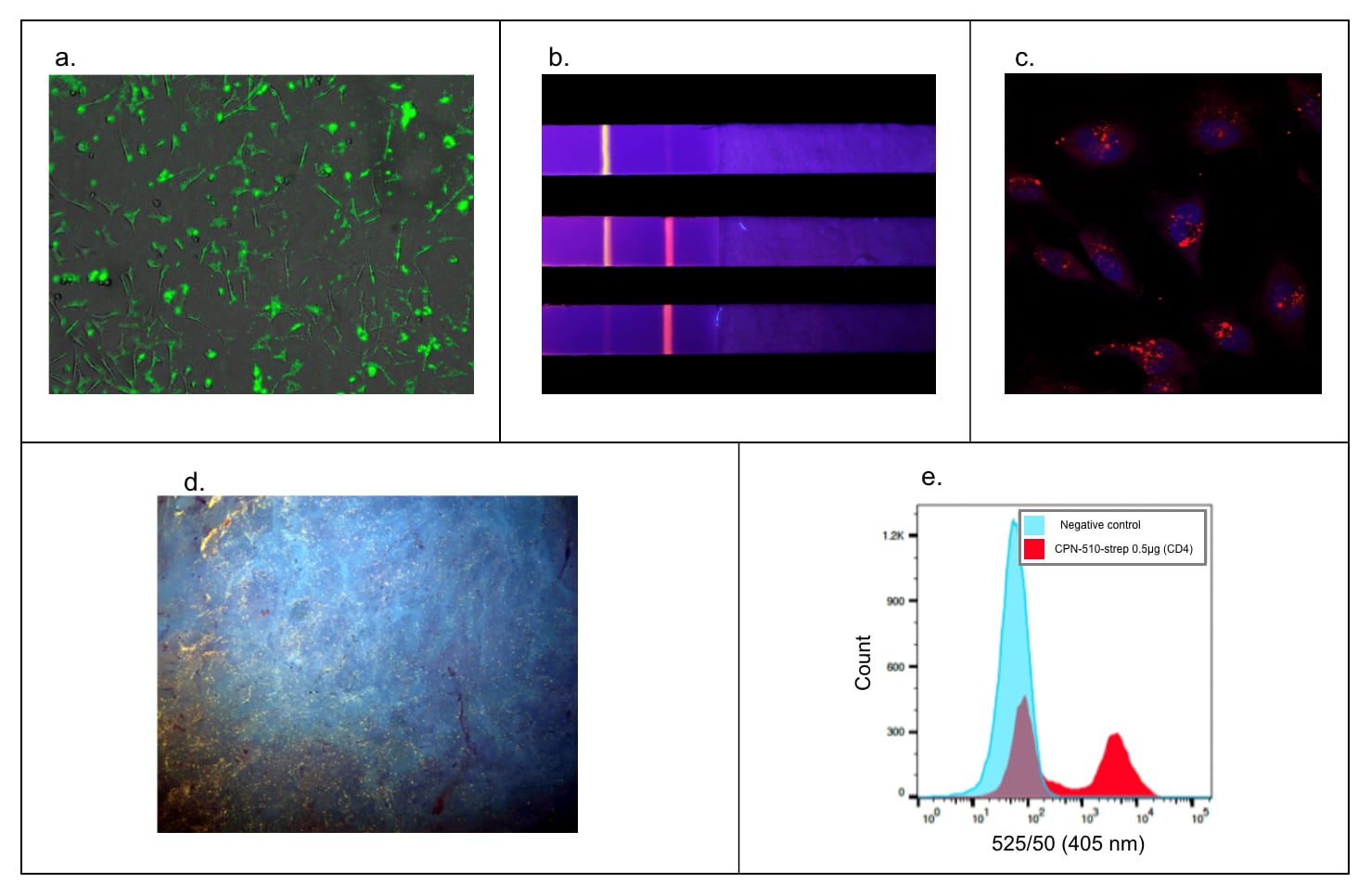
Figure 7.Versatility in a wide range of applications. A) Fluorescence microscopy. HEK293T cell membranes labeled with Cat. No. 905038.9 B) Rapid diagnostic tests with CPN-based rapid diagnostic test strip. Multiple particles can be used simultaneously on one strip for multiple diagnoses.12,13 C) Z-Stack microscopy. HeLa cells stained with Cat. No. 904996 (red) and nuclei stain (DAPI, blue).9 D) Tissue staining using microscopy with UV surface excitation optical section imaging system. Structures can be labeled in paraffin-embedded tissue using Cat. No. 905038 (yellow). E) Flow cytometry. Cat. No. 905038 showing labeling of 50% subpopulation of CD-4 positive cells in flow cytometry.5,9,17
The conjugated polymer core of CPNs provides them with unique photonic properties. This characteristic, coupled with their brightness and photostability, makes CPNs particularly valuable for applications such as two/multi-photon microscopy, where their suitability has been experimentally validated (Figure 8).
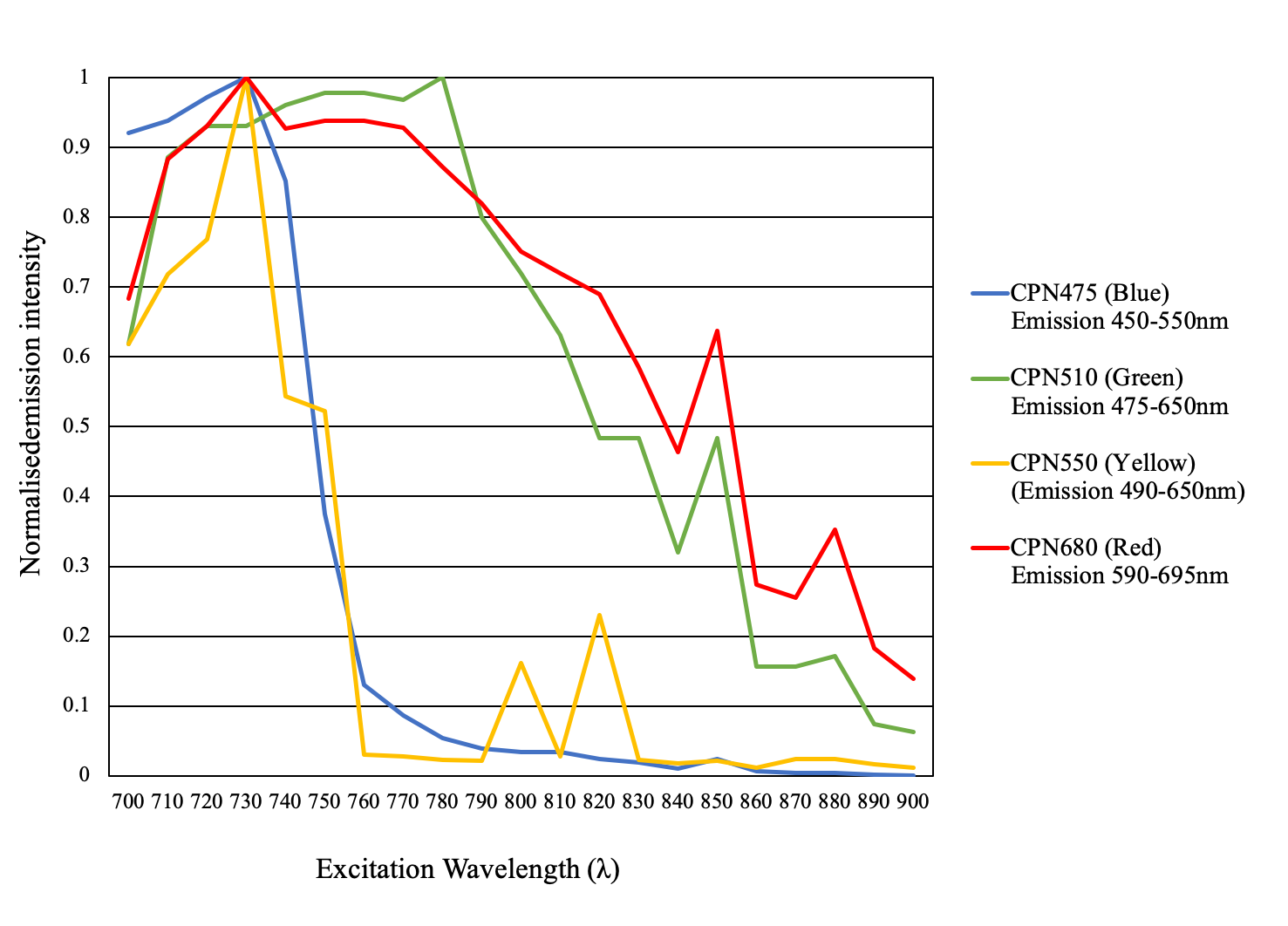
Figure 8.2-Photon microscopy. Excitation 700–900 nm. Emission CPN 475, Cat. No. 905054(blue), CPN 510, Cat. No. 905038 (green), CPN 550, Cat. No. 905046 (yellow), CPN 680, Cat. No. 904996 (red).
Summary
CPNs offer exciting potential for fluorescently labeling molecules and cells. Their intense brightness not only allows for the detection and imaging of low-level proteins and rare cell types but also means that lower levels of excitation can be used sparingly in delicate cells and tissues.3 The robustness of CPNs enables them to be used under high intensity illumination and in extreme experimental conditions if required, while their 24-month shelf-life permits convenient storage at ambient temperatures. Furthermore, they are capable of withstanding temperatures up to 120°C and a pH ranging from 2 to 10. Thanks to their unique brightness, exceptional stability, magnetic capability, and suitability to be conjugated with a wide range of molecules, CPNs offer a multitude of enhancements and benefits for a vast range of life sciences applications.
References
To continue reading please sign in or create an account.
Don't Have An Account?