PYBOX Ligand - Large Binding Site & Rigid Scaffold
William Sommer, Daniel Weibel
Chemfiles Volume 8 Article 2
The PYBOX ligand,1 consisting of a pyridine ring flanked by two oxazoline groups, was introduced in 1989 by Nishiyama.2 PYBOX evolved directly from the BOX ligands. In comparing them, two points merit to be mentioned: (1) the binding site of PYBOXes is big enough to complex even lanthanide cations and (2) the rigidity of the tridentate PYBOX scaffold is increased.
In his very first application, Nishiyama showed that isopropyl-PYBOX 407151 rhodium(III) catalyst effectively promotes the asymmetric hydrosilylation of ketones giving extremely high enantioselectivities (Scheme 1).3
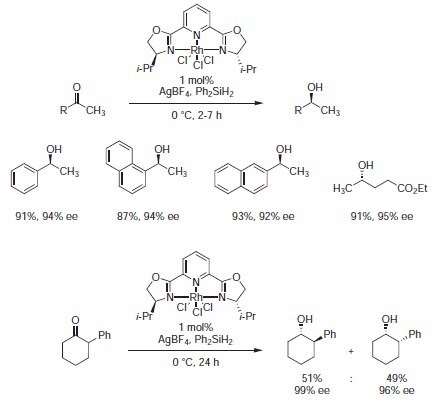
Scheme 1.Catalyst effectively promotes the asymmetric hydrosilylation of ketones giving extremely high enantioselectivities
Li and Wei reported in 2002 the copper(I)-catalyzed enantioselective direct-addition of terminal alkynes to imines. The most successful ligand tested was PYBOX 496073 bearing phenyl groups (Scheme 2).4
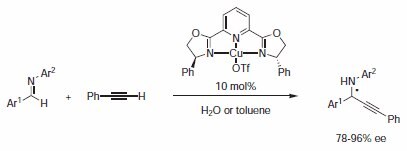
Scheme 2.Catalyzed enantioselective direct-addition of terminal alkynes to imines
Recently, Shibasaki's group reported on lanthanum aryl oxide/PYBOXcatalyzed direct asymmetric Mannich-type reactions using a trichloromethyl ketone as a propionate equivalent donor.5 The La(OAr)3-i-Pr-PYBOX 407151 + LiOAr system gave products in 72 to >99% yield, 8:1 to >30:1 dr, and 92‑98% ee (Table 1).
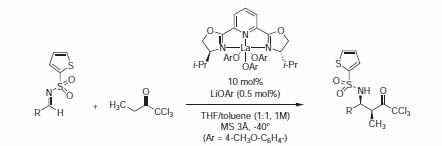
Figure 1.Propionate equivalent donor
The chiral indium(III)-PYBOX complex prepared from indium triflate and C2-symmetric chiral PYBOX 673986 was found to be an effective chiral ligand for the enantioselective allylation reaction of aldehydes with allyltributylstannane, giving high enantioselectivities.6 Using this methodology, the chiral steroidal aldehyde could be allylated with excellent enantioselectivity ((22S):(22R)= >99:1) and in good yield (78%). Furthermore, the reaction was highly chemoselective, reacting only with the aldehyde functionality. No reaction was observed with the enone functionality in the A ring (Scheme 3).
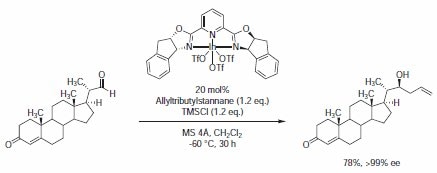
Scheme 3.The chiral indium(III)-PYBOX
References
To continue reading please sign in or create an account.
Don't Have An Account?