Fractional Precipitation Protocol for Affinity Chromatography Samples
Fractional precipitation is frequently used at laboratory scale to remove gross impurities from small sample volumes and occasionally used in small-scale commercial production. Precipitation techniques separate fractions by the principle of differential solubility. Because protein species differ in their degree of hydrophobicity, increased salt concentrations can enhance hydrophobic interactions between the proteins and cause precipitation. Fractional precipitation can be applied to remove gross impurities in three different ways, as shown in Figure 75.
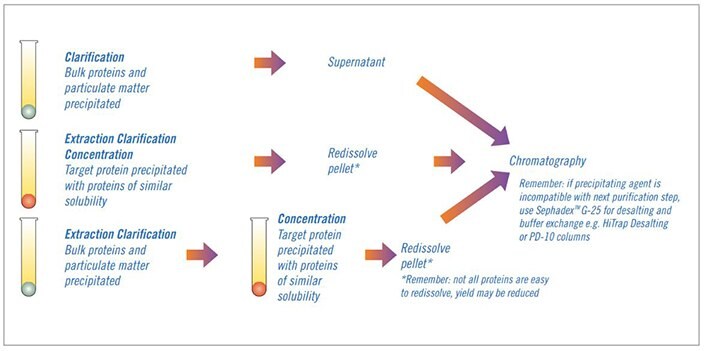
Figure 75.Three ways to use precipitation.
Details taken from:
Scopes R.K., Protein Purification, Principles and Practice, Springer, (1994), J.C. Janson and L. Rydén, Protein Purification, Principles, High Resolution Methods and Applications, 2nd ed. Wiley Inc, (1998).
Personal communications.
Ammonium Sulphate Precipitation
Some proteins may be damaged by ammonium sulphate. Take care when adding crystalline ammonium sulphate: high local concentrations may cause contamination of the precipitate with unwanted proteins.
For routine, reproducible purification, precipitation with ammonium sulphate should be avoided in favor of chromatography.
In general, precipitation is rarely effective for protein concentrations below 1 mg/mL.
Solutions needed for precipitation:
- Saturated ammonium sulphate solution (add 100 g ammonium sulphate to 100 mL distilled water, stir to dissolve).
- 1 M Tris-HCl, pH 8.0.
- Buffer for first purification step.
- Filter (0.45 µm) or centrifuge the sample (10 000 g at +4 °C).
- Add 1 part 1 M Tris-HCl, pH 8.0 to 10 parts sample volume to maintain pH.
- Stir gently. Add ammonium sulphate solution, drop by drop. Add up to 50% saturation*. Stir for 1 hour.
- Centrifuge 20 minutes at 10 000 g.
- Remove supernatant. Wash the pellet twice by resuspension in an equal volume of ammonium sulphate solution of the same concentration (i.e. a solution that will not redissolve the precipitated protein or cause further precipitation). Centrifuge again.
- Dissolve pellet in a small volume of the buffer to be used for the next step.
- Ammonium sulphate is removed during clarification/buffer exchange steps with Sephadex G-25, using desalting columns (see page 133, Buffer exchange and desalting for affinity chromatography).
*The % saturation can be adjusted either to precipitate a target molecule or to precipitate contaminants.
The quantity of ammonium sulphate required to reach a given degree of saturation varies according to temperature. Table 14 shows the quantities required at +20 °C.
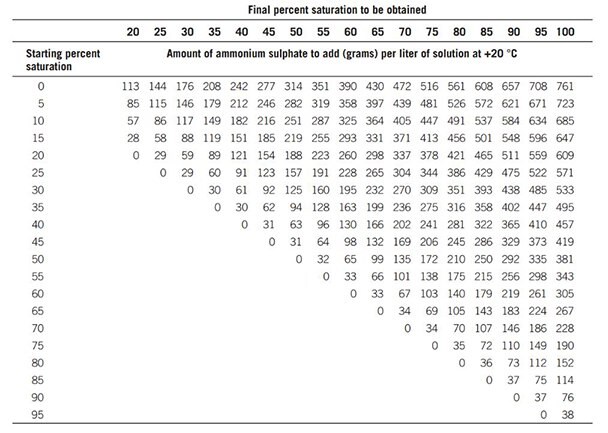
Table 14.Quantities of ammonium sulphate required to reach given degrees of saturation at +20 °C.
To continue reading please sign in or create an account.
Don't Have An Account?